The enzyme-linked lectin assay (ELLA) was introduced in 1983 by McCoy et al. (. McCoy et al., 1983 ) as a variation of the more famous enzyme-linked immunosorbent assay (ELISA). The methodology was adapted to the evaluation of binding properties of lectin ligands, as a valid alternative to the widely used HIA. ELLA is a quantitative technique of higher sensibility compared to the hemagglutination one, while still relatively cheap. Working volumes are conveniently small, in the 25-250 µL range. The use of multi-well plates is suitable for the analysis of multiple samples simultaneously. Moreover, the method is readily applicable to ELISA microtiter plate readers without the need for any adjustment. A reference saccharide is immobilised on the bottom of the microtiter plate in a standard ELLA experiment to mimic the real situation in which a lectin encounters its ligand(s) on the cellular surface. The solid phase can consist of a glycoprotein or polysaccharide coating. Frequently a covalent immobilisation of synthetic carbohydrates on a polymeric surface (e.g. polyacrylamide) is preferred. The chemical ligation of structurally defined ligands allows us to have control over density, accessibility and topology of glycan presentation. These aspects are rather crucial for overcoming problems of micro-heterogenicity since the assay has shown to be sensitive to the nature of the matrix (Maierhofer et al., 2007; Andre et al., 2001).
Then, the competition in binding the lectin of interest is set up between mono- or a multivalent glycan in solution and the ligand fixed on the surface. The ability of the compound in solution to inhibit the adhesion of the lectin to the bottom of the well is finally measured by optical spectroscopy. Although conceptually close to the ELISA method, ELLA does not require the use of antibodies for detection. On the contrary, an enzyme is directly conjugated to the lectin for colourimetric visualisation. The most common detection system relies on labelling the lectin with the HRP (horseradish peroxidase) enzyme through biotin-streptavidin conjugation (Fig. 8). HRP is extensively used in biochemistry applications since it catalyses the conversion of numerous substrates in coloured products that are measurable by UV-Vis spectroscopy. For its extensive use in research, HRP is easily accessible and affordable. However, the same cannot be said about the whole lectin-HRP system, since many lectins are not commercially available in the conjugate form. On the other hand, the lectin of interest is often produced and purified in-house before any type of assay is performed. Proteins are routinely expressed in biotinylated form or can be chemically linked to biotin afterwards.
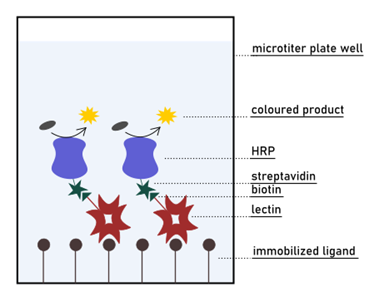
Along with the need for protein labelling and ligand immobilisation, the requirement of washing steps also makes ELLA a time-consuming and challenging assay. Careful removal of unbound lectin after an incubation period is fundamental. Repeated washes assure that the signal is generated only by the fraction of lectin that is bound to the surface. If the set-up of the assay is correct, the signal will decrease in the presence of a more potent lectin inhibitor. This because the intensity of colourimetric reaction inversely correlates with the amount of lectin interacting with such compound. In other words, the development of colour is inversely proportional to the affinity of the ligand in solution.
As a measure of potency, the value of IC50 can be obtained as the inhibitor concentration that reduces the original signal by 50%. It is important to remark that seldomly full inhibition has been observed. Noticeably, maximal inhibition was often reported to be significantly lower than 100%. Poor IC50 reproducibility is indeed a drawback of ELLA. Even though the IC50 value obtained by curve fitting is reliable, high variability is observed in the results obtained in different laboratories on identical systems. Such disadvantage underlines a remarkable dependence of the assay outcome on the experiment set-up in its delicate phases (conjugations and washing) again. Per contra, working on a heterogeneous surface has the advantage of effective mimicking in the biological environment. As such, it guarantees a representative study on the ability of synthetic compounds to inhibit real binding modes on a multivalent surface.
The ELLA technique is a standard tool for evaluating the activity of monovalent ligands, and it has been applied for multivalent glycosides as well (Kohn et al., 2004). Under the ELLA working conditions, lectin aggregation by potent multivalent binders should be hampered by the presence of the 40kDa HRP protein. However, cross-linking phenomena can still potentially take place. The formation of aggregates on the surface of the sample well should always be checked, as to avoid the generation of false-negative results.
