Isothermal Titration Calorimetry (ITC) is considered the method of choice to analyse protein-carbohydrate interactions. It allows the precise determination of the thermodynamic parameters associated with the binding process (ΔG, ΔH, ΔS, Kd) in a single experiment. This technique consists of titrating a protein solution with that of a ligand, or vice versa. Immediately after the addition, the energy that is released (or absorbed) upon the formation of the protein-ligand complex is measured by the apparatus. The calorimeter detects the temperature unbalance between the sample and the reference cell, consequently applying thermal power to compensate for such difference. The signal generated while restoring thermal balance is displayed as one peak for each injection. Peak integration provides information about the heat exchange associated with the binding event during that specific injection. The ITC technique has been reviewed as a powerful tool to obtain enthalpy/entropy determinants during the formation of both monovalent and multivalent carbohydrate-lectin associations (Velazquez et al., 2004; Dam et al., 2016).
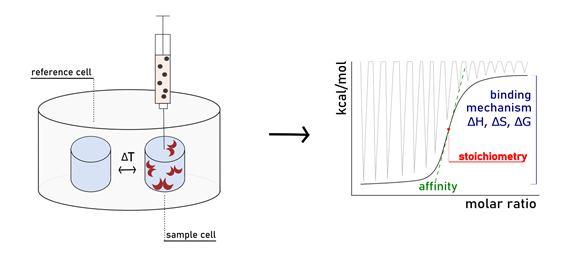
In addition to the thermodynamic framework, the stoichiometry value (n) for the complex is also obtained by ITC. Knowing precisely the stoichiometry of the observed binding process is of crucial importance for its study. The n value in a monovalent interaction can serve to confirm the number of active binding sites of a protein. In the contest of multivalence, particularly interesting is that the exact number of epitopes participating to the interaction (i.e. functional valence, v) is not always equal to the actual number of epitopes present in the structure (i.e. structural valence). Dam et al. were the firsts to refer to the concept of functional valence in intermolecular multivalent binding. The same authors elaborated the equation v=1/n (where n is the stoichiometry value obtained directly from the ITC experiment) to calculate functional valence. They exemplified their theories while studying the cross-linking of ConA by a synthetic tri-mannoside. Even if their glycoside is structurally trivalent, the n value obtained by ITC was 0.5, indicating that the compound is functionally bivalent in binding to ConA (Dam et al., 2002).
Stoichiometry is essential since it determines the structure of cross-linking aggregates and could serve as a controlling mechanism for lectin-mediated signal transduction (Dam et al., 2017). The association of reactants in such supramolecular assemblies can also result in insoluble materials that are detrimental for the correct execution of the ITC measurement. Dilution of the samples can be a solution in such situations, but overcoming this problem is not always so trivial. The operator has a low margin of action since volumes of syringe and sample cell are fixed. At the same time, molar ratio and protein concentration must stay within a specific range to assure the generation of a complete binding isotherm.
Another critical aspect of the technique is the need for consistent amounts of lectin and glycoside ligand. In a VP-ITC instrument, the typical size for the sample cell is 2 mL, titrated by 0.5 mL of the ligand. These are significant volumes if compared with the ones required by assays that work in the same or lower concentration range. However, more modern instrumentation, such as MicroCal iTC200, allow lower sample consumption and automation. Finally, the heath of binding can be altered by non-specific events, such as dilution, changes in pH or ionic strength. Consequently, a perfect match between buffers must be assured to not incur in non-specific effects.
Surpassed these complications, isothermal titration calorimetry remains the first method of choice when there is a need for an in-depth understanding of the forces guiding a binding process. Even though ITC was for long confined as a solely thermodynamic technique, calorimetry data inherently contain the kinetic information too. The signal is expressed as heat power in function of time, therefore allows the calculation of kinetic kon and koff of the observed process. A software extension of ITC, named kinITC, was elaborated to obtain those constants. The theoretical basis and examples of its application can be found elsewhere (Pineiro et al., 2019; Dumas et al., 2016; Burnouf et al., 2012; Li et al., 2017.). Today, a synergic determination of kinetic and thermodynamic parameter is possible within a single ITC measurement.