When studying the role of lectin in biology or when assessing the capability of new therapeutics in (dis)regulating these functions, the choice of an assay suitable for this purpose is of absolute importance. Along with the correct set up of a carbohydrate-protein binding assay, an appropriate interpretation of the outcome of such experiments is a necessity to avoid misleading interpretations.
The successful development of synthetic multivalent systems and the optimisation of their design principles are strongly interconnected with the characterisation of their biological effect. To yield meaningful results, the features of the assay have to reflect as much as possible the real situation in which the compound is to be used. For example, we might consider different test settings if we want to assess the capability of a multivalent compound to bind a single lectin on the surface of a pathogen, or if instead, we want to evaluate its ability to aggregate more receptors present on the cellular membrane.
When discriminating between all the techniques available, an evident difference is the homogeneous or heterogeneous nature of the assay. We can distinguish assays that operate in solution from the ones in which one binding partner is immobilised on a surface. This crucial difference must be considered when comparing results obtained by different means. In effect, the correlation of results generated by different platforms is generally not advisable: since each experiment relies on the detection of different properties, it gives inevitably different outcomes. Because of this, testing more compounds for a certain desired effect gives meaningful data when the same technique is used for analysing all of them. Nevertheless, using assays based on different principles to answer the same question is valuable when looking at the general trend of the results. Provided that same interaction conditions are maintained, relative potencies for a set of ligands should be in principle confirmed, no matter the method to analyse them.
In designing lectin inhibitors, biochemists typically look at decreasing the Kd of binding as a sign of potency. The dissociation constant at equilibrium conditions is an absolute parameter that allows accessible and unequivocal determination of the strength of an interaction between lectins and their ligands. To calculate the Kd for a protein-ligand complexation, the concentration of free and bound reaction partners must be known (either directly or indirectly). The measurement of affinity can become arduous when the Kd is in the picomolar or nanomolar range, and the sensibility of the assay also has to be investigated.
The knowledge of thermodynamic parameters that contribute to the free energy of binding is essential for the iterative optimization of the molecular structures under scrutiny. However, it can be speculated that the estimation of Kd in vitro does not efficiently predict the behaviour of a drug candidate in vivo. If the synthetic compound will have to compete with a natural ligand to reveal its therapeutic qualities, the measure of inhibitory potency (in terms of IC50) in a competitive binding assay is more informative then the mere thermodynamic profile.
Another possible way to look at the interaction involves the study of kinetic parameters. In the kinetic perspective, high avidity effect can be reached in out-of-equilibrium conditions, for example when a strong association continues without complete dissociation via the kinetically trapped state (Lanfranco et al., 2019; Li et al., 2014). The dissociation rate (koff)) of the process in observation is a crucial parameter to measure the time a drug spends in contact with its biological target, frequently expressed as residence time Ƭ=1/koff. This kinetic parameter has been recognized as a critical element for drug optimization since it reflects consistently the duration of efficacy in vivo and target selectivity.(Tonge, 2018; Bernetti et al., 2019)
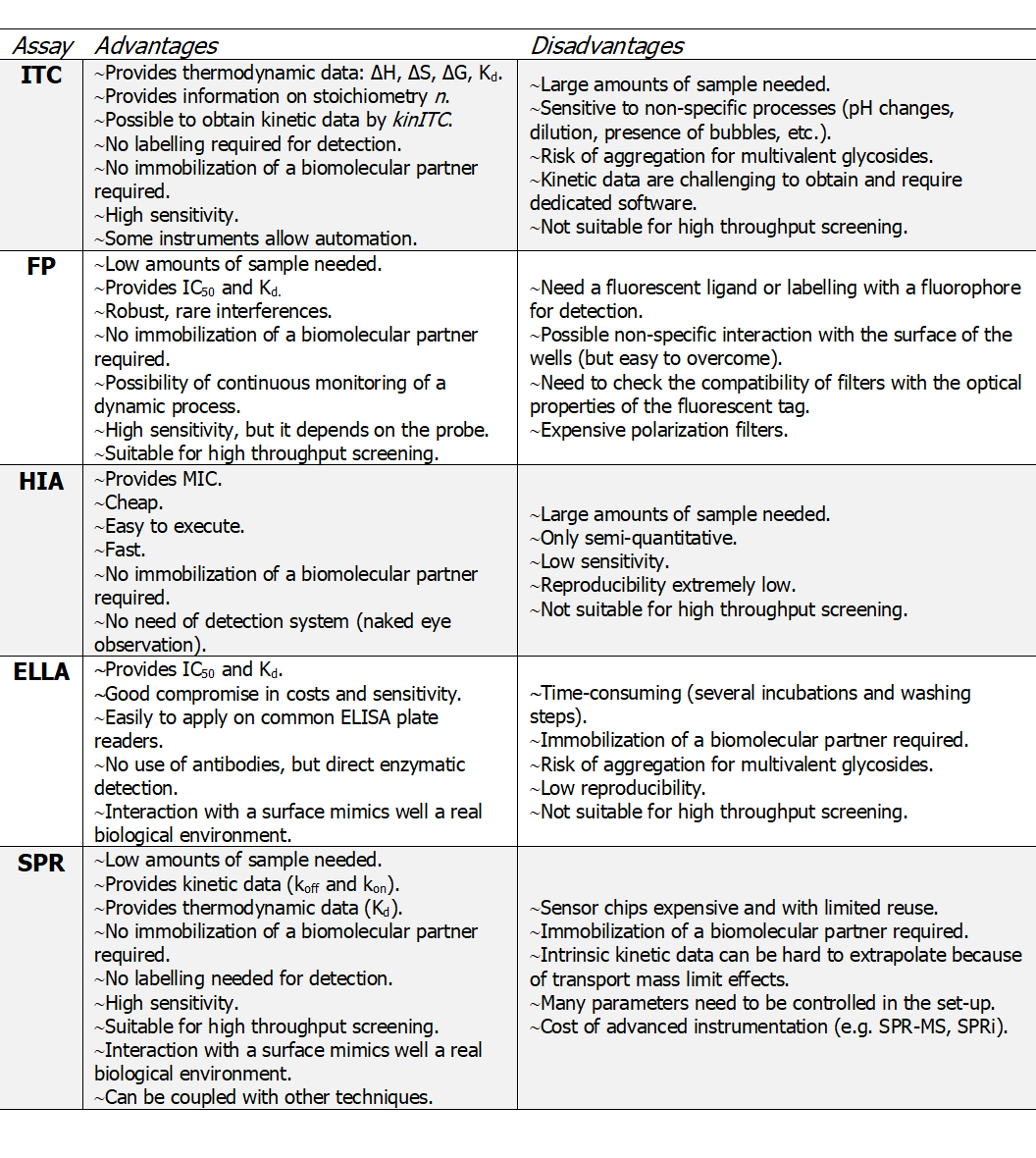
Now, not all of this information can be obtained within the same assay. A careful choice of the method for studying lectin-sugar interaction is necessary to get valuable results, and it must be selected on a case-by-case basis. In the next paragraphs, the most common types of assay used in glycoscience will be presented in detail. We will underline their respective advantages and limits to help the readers in choosing the best technique for their purpose (Table 1).