In the early history of lectin research, the most common method to identify lectin activity was to check the ability to agglutinate erythrocytes. In the past, lectins were referred to as “agglutinins” because of their ability to cause cell agglutination through binding the sugar residues present on the surface. Nowadays, it is known that such nomenclature is a generalization since not all the lectins are agglutinins, and not all of the agglutinins are lectins (also antibodies have this property).
Even though the hemagglutination assay (HA) is almost a century old (Summer & Howell, 1936; Hirst, 1942), it remains a fast and easy assay to determine lectin specificity. Originally, observations were made on the capacity of lectins to distinguish types of cells. Erythrocytes from various species are widely used in the hemagglutination assay, most commonly chicken, rabbit, pig, or human erythrocytes. The test is based on the observation that red blood cells aggregate in the presence of the lectin, producing a cross-linked network in suspension throughout the sample. This happens according to cell type and animal origin, revealing a specific recognition of the glycans decorating the cell membrane by the lectin under
observation.
The glycan specificity was determined, in early research, by semi-quantitative hemagglutination-inhibition assay (HIA). Being a rapid and low-cost technique, HIA is still performed nowadays as an initial filter for more informative studies. Glycans are added to the mixture of lectin and erythrocytes in increasing concentration to perform this test. When the formation of the cross-linked matrix is prevented entirely, the inhibitory potential of the glycan can be determined (Fig 7). (Sano & Ogawa; 2014) This potency is expressed in terms of minimum inhibitory concentration (MIC) for a given ligand.
The amounts of protein and ligand required for the assay are high. Usually, it is necessary to work at a high micromolar range of concentration to generate a significant effect. Such a requirement makes HIA not a suitable approach for the evaluation of potencies of precious compounds, such as glycomimetics or multivalent glycosides obtained after intense synthetic efforts.
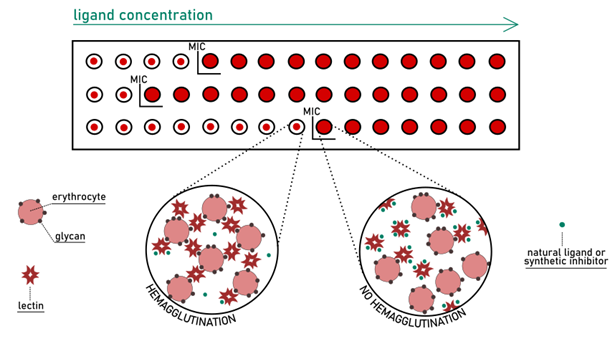
The HIA method, while quick, cheap, and easy, suffers from poor reproducibility. First, the determination of MICs is the result of a naked-eye observation. Sometimes the interpretation of the various levels of partial agglutination can be difficult to classify in a positive or negative value. Secondly, the selection of erythrocytes is critical: the glycomic profile on erythrocytes varies between species, and the quality of samples can fluctuate substantially even within the same species. The weakness of the assay makes it challenging to compare results generated from separate experiments. Problematic is also the attempt to extract quantitative information that goes beyond the approximate order of magnitude of binding affinity.
The hemagglutination assay historically remains the method of choice for the identification of new multivalent lectins. However, it is not informative of the kinetic/thermodynamic parameters of ligand-lectin interaction. Rather than providing insights on the strength of binding, it indicates the ability of glycodendrimers or monosaccharides to drive/inhibit aggregation processes. The introduction of more advanced techniques in glycoscience allows today a better understanding of lectin-carbohydrate interactions on the molecular level.
