The numbering of Human Siglecs follows the order of their discovery. The representatives murine Siglecs that are not homologous to the human ones are designed by a letter (e.g. Siglec-F). (Varki et al., 2015) Human Siglecs are divided into two groups depending on their homology and evolutionary conservation (Figure 2). CD22-related Siglecs share only 25-30% of sequence identity, and they have true orthologs in other mammalian species: Sialoadhesin (Siglec-1), CD22 (Siglec-2), myelin-associated glycoprotein (MAG, Siglec-4), and Siglec-15 belong to this group. The second group consists of CD33-related Siglecs (CD33rSiglecs). Among these, Siglec-12 lost its ability to recognize sialic acid, and it was renamed Siglec-XII, while the Siglec-13 gene is not present in humans; therefore, they will not be discussed in the following sessions. (von Gunten & Bochner, 2008) CD33rSiglecs are characterized by high sequence homology and minor conservation between species.(Angata et al., 2004; Bornhöfft et al., 2018; Crocker et al., 2007) CD33rSiglecs are also expressed in mice, but they represent paralogs and not true orthologs of the human counterparts: Siglec-F and Siglec-G correspond to Siglec-8 and Siglec-10, respectively. (Jandus et al., 2011) The high evolutionary differentiation might respond to different mechanisms of evasion from the immune system used by pathogens or cancer cells, like mimicking our self-glycans.(Angata, 2006; Macauley & Paulson, 2014; Varki & Angata, 2006)
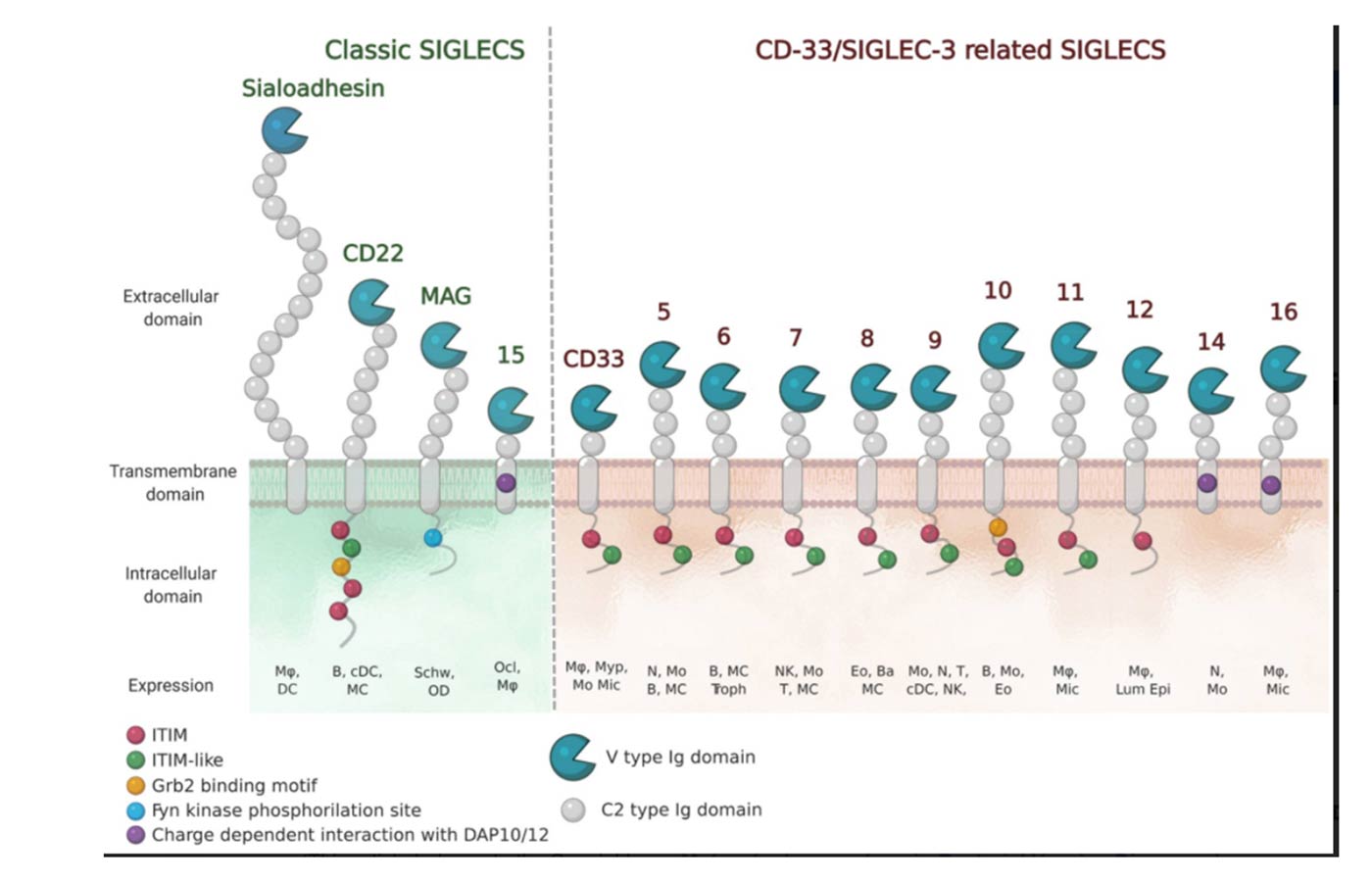
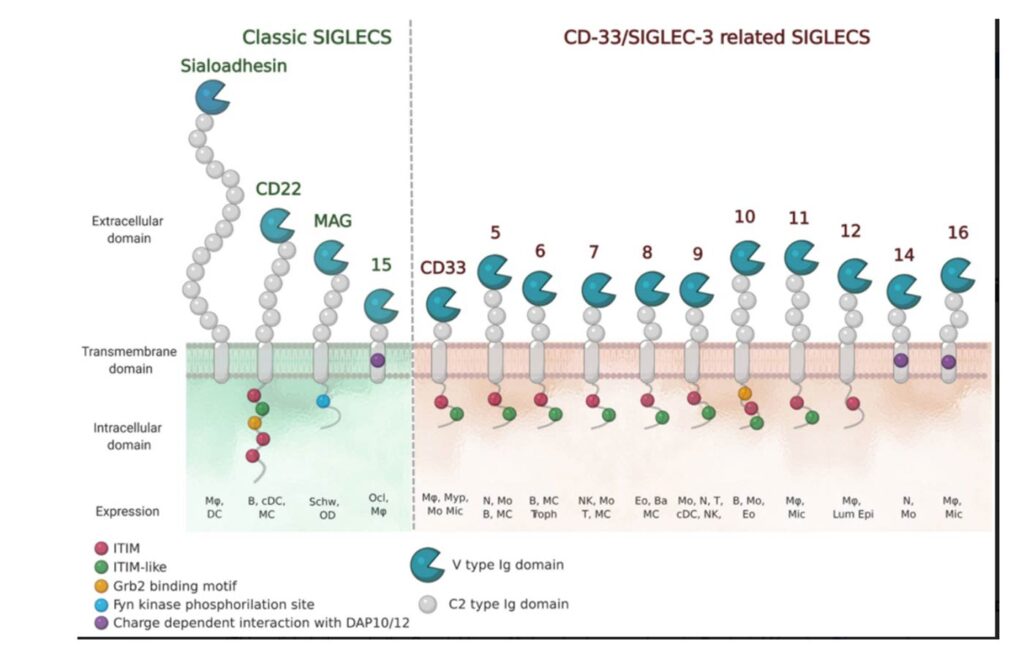
Structurally, Siglecs present a V-set domain on their extracellular N-terminal part, containing the sialic acid-binding pocket, and a variable number of C-2 set domains (from 1 to 16), both immunoglobulin (Ig)-like domains. In their resting state, Siglecs are generally engaged in cis-interactions with sialic acid ligands expressed on the same cell surface. Sialoadhesin is an exception : due to its length (16 C-2 set domains) it is mainly involved in trans-interactions. (Crocker et al., 2007 ; Hartnell et al., 2001 ; Nakamura et al., 2002) High-affinity ligands can compete with the cis-ligands and bind to Siglecs, as shown with CD22. (Collins et al., 2006)
The signaling is initiated by the intracellular regulatory motifs, except for Sialoadhesin that does not contain any domain in this region : most of the Siglecs present an immunoreceptor tyrosine-based inhibitory motif (ITIM). Src kinases first phosphorylate these domains, then, Src-homology 2 domain (SH2)-containing phosphatases SHP-1 and SHP-2, once recruited, de-phosphorylate important signaling molecules and initiate the inhibitory cascade. (Pillai et al., 2012) Only Siglecs-14, -15 and -16 have an activating activity. They contain positively charged amino acid residues that bind to the coreceptor DAP12, which in turn contains an immunoreceptor tyrosine activation motif (ITAM) in its cytoplasmic domain. Once the binding to Siglecs takes place, DAP12 initiates the activating signaling. (Cao et al., 2008a ; Takamiya et al., 2013) Generally, activating Siglecs are coupled with inhibitory counterparts : Siglec-5 vs Siglec-14 and Siglec-11 vs Siglec-16, usually expressed on the same cells. (Macauley et al., 2014)
As previously mentioned, all Siglecs bind sugars containing sialic acid, but each with its specificities. They have evolved to recognize all the different ways in which the sialic acids are displayed on glycoproteins and glycolipids to be specific for the type of linkages (α2-3, α2-6, and/or α2-8), or the type of sugars to which the sialic acid is bound to. Usually, Siglecs bind to their natural sialoside ligands with low affinities (0.1-1 mM). (Blixt et al., 2003) The N-terminal V-set domain mediates the binding. It is composed of antiparallel β-sheets with a shallow binding pocket for the sugar and an additional loop in the proximity, which provides more specificity for carbohydrates. One of the most conserved amino acids in this region is an arginine residue which interacts with a salt bridge with the carboxylate of the sialic acid. (Movsisyan & Macauley, 2020)
Siglecs occur mostly on immune cells, except for MAG, which is present on myelinating cells of the nervous system. Depending on the roles and sites of expression, Siglecs are involved in many physiological and pathological processes, especially as immuno-modulators. (Büll et al., 2016)