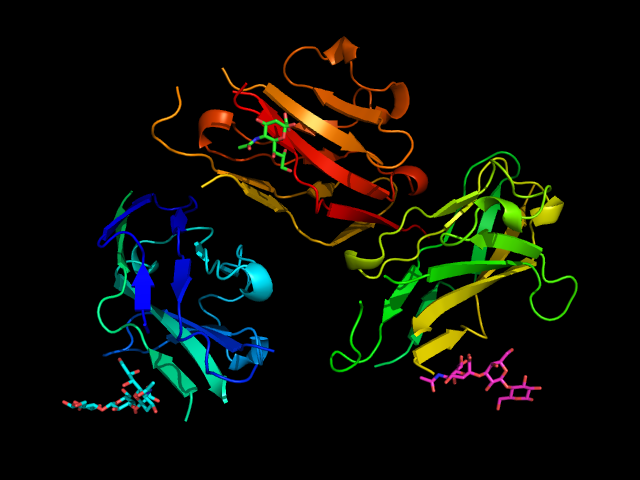
Sialoadhesin (also known as Sn, Siglec-1 or CD169) was one of the first proteins of the Siglec family to be characterized.(Crocker et al., 1994; Kelm et al., 1994) It shows a high level of conservation between mouse and human, especially in the extracellular region. It is the only Siglec that presents 17 Ig-like domains outside the cell, including one V-set domain and 16 C-2 set domains. (Crocker et al., 1991; Hartnell et al., 2001) Unlike the majority of Siglecs, it does not have an intracellular domain. Due to the length of the extracellular portion, Siaoloadhesin is more available for trans-interactions with external ligands than cis-interactions with cell-surface molecules. For this reason, it has a principal role in cell-cell interactions and signaling. (Munday et al., 1999; O’Neill et al., 2013)
Sialoadhesin occurs mainly on macrophages (described as CD169+ macrophages), playing a crucial role in host protection. Upon recognizing sialic acid epitopes expressed on the cell surface of bacteria or viruses, Sialoadhesin promotes the phagocytic activity of macrophages, optimization of antigen presentation, and following activation of the adaptive immune response. (Chang et al., 2014; Chang & Nizet, 2020; Klaas et al., 2012)
It presents the same role on other antigen-presenting cells (APC) like dendritic cells (DC), where it triggers antiviral immunity taking part in pathogen uptake via endocytosis. Unfortunately, enveloped viruses such as Ebola or human immunodeficiency virus (HIV)-1 have exploited this mechanism to enhance the auto dissemination in tissues and escape the immune system.(Gummuluru et al., 2014; Perez-zsolt et al., 2019; Perez-Zsolt et al., 2019) HIV’s capture by DC facilitates virus dissemination and release in cell contact sites with the CD4+ T cell. Therefore, the virus can easily reach these sites, mainly present in lymphatic tissues and trans-infect T-cells. (McDonald et al., 2003; Sewald et al., 2015)
Sialoadhesin has also been identified as a potential biomarker for autoimmune diseases: for example, its expression increases in some pathologies correlated with interferon signaling as systemic lupus erythematosus or systemic sclerosis.(Biesen et al., 2008; Eakin et al., 2016; York et al., 2007) In particular, Sialoadhesin is associated with an increased expression on peripheral blood monocytes in rheumatoid arthritis. (Xiong et al., 2014)
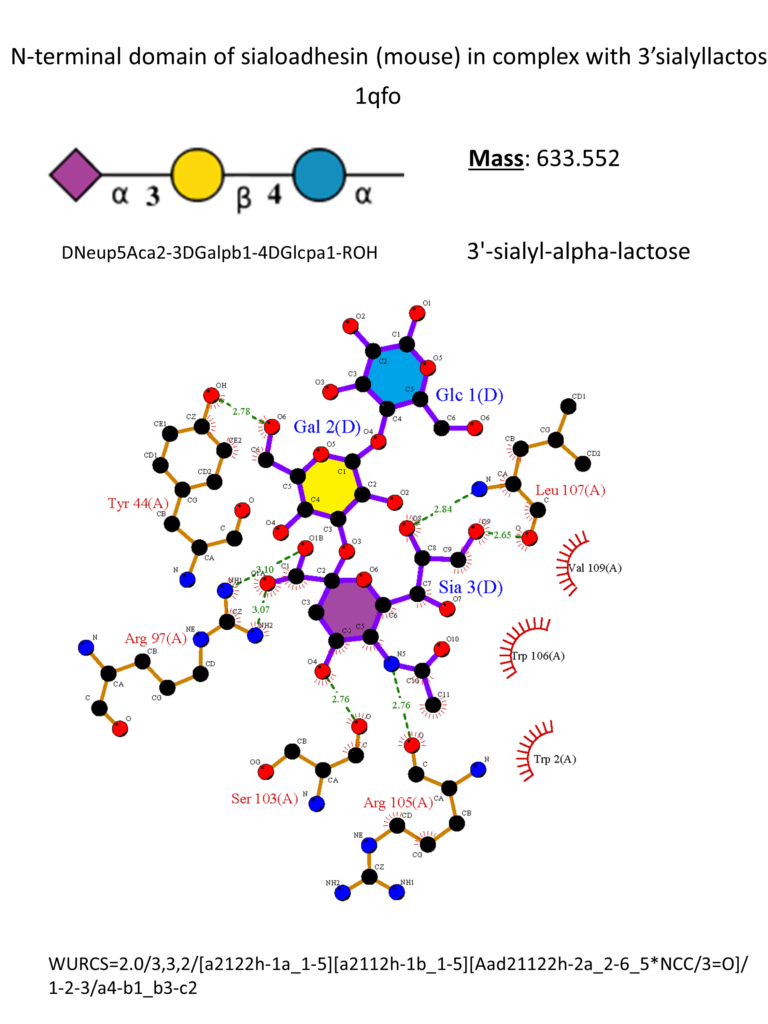
Sialoadhesin has a preference for α2-3 glycosidic linkage. (Crocker et al., 1999) The crystal structure of the N-terminus of Sialoadhesin together with the 3’-sialyllactose was firstly reported in 1998. The sialic acid almost entirely mediates the binding, and a salt bridge between its carboxylate and Arg97 is essential for the binding. (May et al., 1998) Other essential interactions include one hydrogen bond between OH-4 and the main chain carbonyl of Ser103 and a second one between the amide nitrogen and the main chain carbonyl of Arg105. Hydroxyl groups in positions 8 and 9 form hydrogen bonds with the backbone amine and carbonyl groups of Leu107, respectively. Hydrophobic interactions occur mainly with Trp106 (Figure 3). (May et al., 1998)
The development of small molecules targeting Sialoadhesin started from the methyl-α-Neu5Ac, which displays an affinity in the 1-3 mM range. The subsequent introduction of biphenyl substituents in position 9 of the sialic acid improved the affinity of 13-fold thanks to hydrophobic interactions in a pocket close to the sialic acid-binding site (biphenylcarbamoyl(BPC)-Neu5Ac 2, Figure 4). (Zaccai et al., 2003)
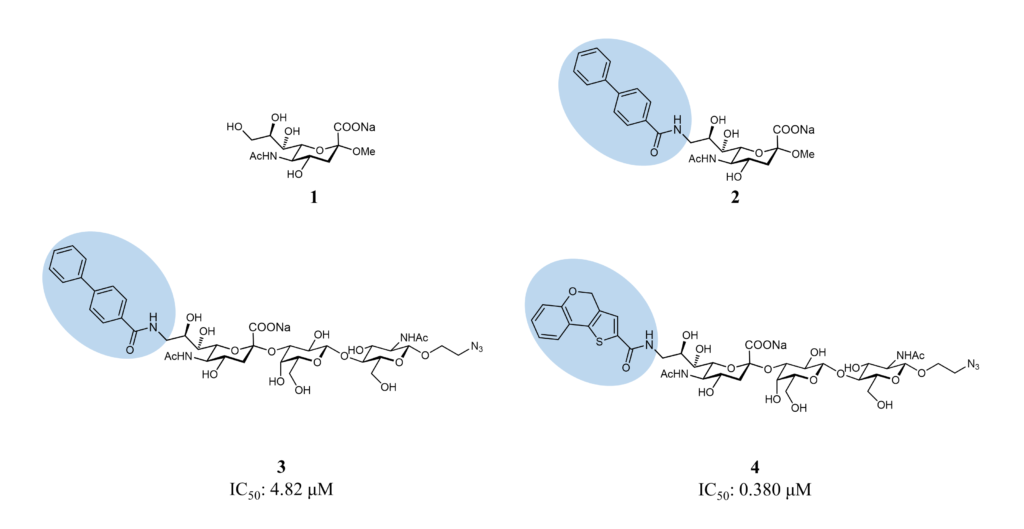
Subsequently, liposomes decorated with a related ligand, the 9-N-BPC-Neu5Acα2-3Galβ1-4GlcNAc (3, Figure 4), could target Sialoadhesin-expressing cells, but the interaction was not selective enough for in vivo targeting. (Blixt et al., 2003) As a result of these promising results, a virtual screening focusing on position 9 was performed to improve the affinity and selectivity in this region: the best compound identified, the 9-N-(4H-thieno[3,2-c]chromene-2-carbamoyl)-Neu5Acα2-3Galβ1-4GlcNAc 4 (TCC-Neu5Ac, 4, Figure 4) bound selectively to Sialoadhesin with an IC50 of 0.38 μM, measured by competitive binding assay. The same compound, displayed on liposomes, targeted efficiently in vivo CD169+ macrophages.(Edgar et al., 2019; Nycholat et al., 2012) These liposomes can represent an optimal tool to deliver antigens or drugs to macrophages selectively.