Siglec-5 and Siglec-14 are two members of the Siglecs family pair receptors. They occur on the same cells, playing different roles: while Siglec-5 is an inhibitory receptor, Siglec-14 is an activator. Similar to the other Siglecs, the inhibitory activity is associated with the ITIM motif in the intracellular region. Siglec-14 is paired with the activating receptor DAP. Their extracellular domains have the same amino acid sequence in the first two Ig-like domains, showing preferences for the same ligands.(Angata et al., 2006 ; Cornish et al., 1998) Siglec-5 and Siglec-14 occur on neutrophils, macrophages and monocytes. (Lock et al., 2004)
Some pathogens can exploit Siglec-5 to escape the immune response. For example, group B Streptococcus (GBP) uses a membrane-anchored β-protein to bind Siglec-5 that, being an inhibitory receptor, causes the inactivation of the immune cells. (Carlin et al., 2009) The pairing to Siglec-14 and its activating activity can trigger the immune response. From an evolutionary perspective, the development of a pairing mechanism may have been a response to pathogens. (Ali et al., 2014) Siglec-14 can also interact with H. influenza in a sialic acid-dependent manner, stimulating the proinflammatory activity of macrophages, exacerbating the inflammation states, and increasing the chances to cause chronic obstructive pulmonary disease (COPD). (Chang & Nizet, 2014) The V-set and C2-type Ig domains of Siglec-5 have been crystallized in complex with two sialylated carbohydrates, the α2-3- and α2-6-sialyl lactose (Figure 11). The interactions are quite weak, in the low mM range.
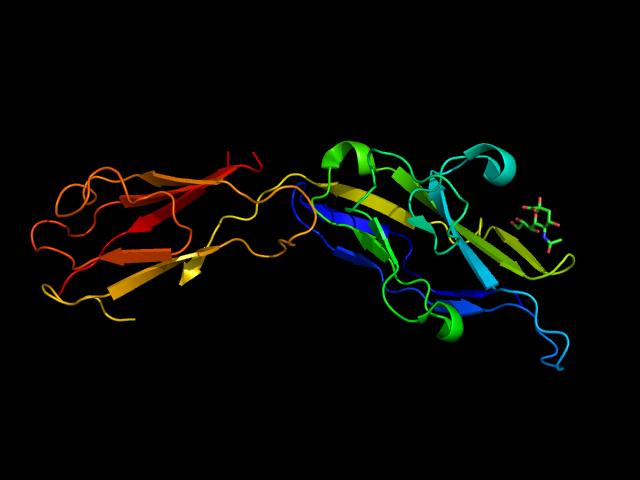
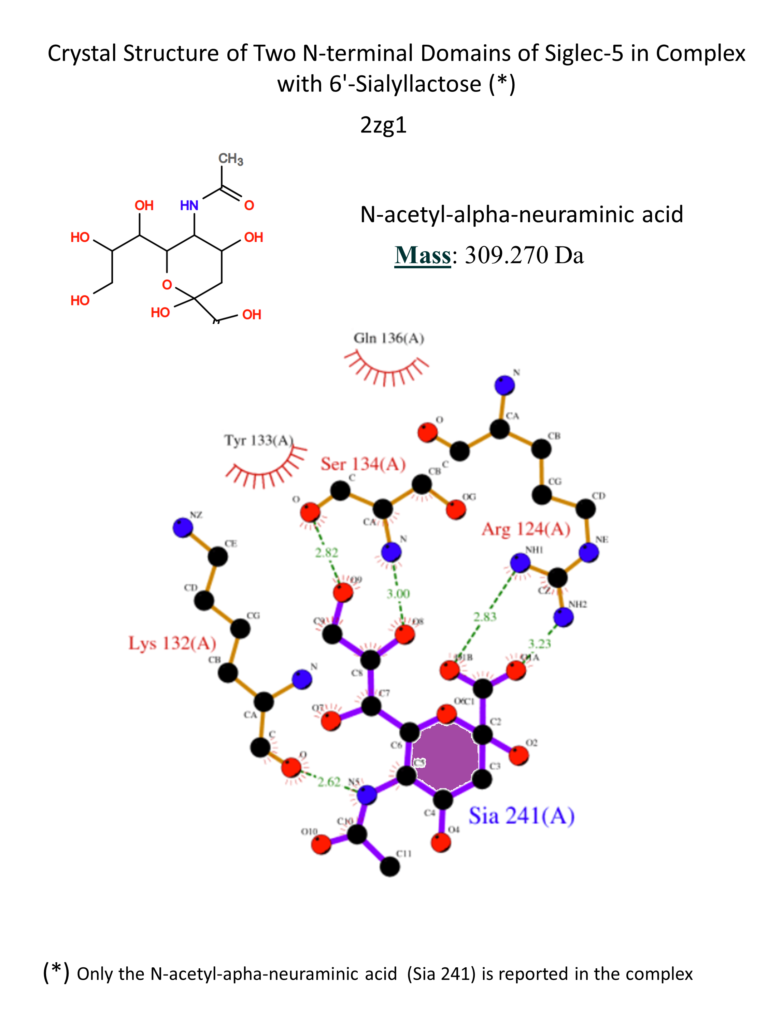
The main interactions involve a salt bridge between the carboxylate of the sialic acid and the conserved Arg124, hydrogen bonds between Lys132 and the amide in position 5 and between Ser134 and the hydroxyls in position 8 and 9. Fewer contacts involve the lactose part of the ligand. (Movsisyan & Macauley, 2020; Zhuravleva et al., 2008)
A few Siglec-5/Siglec-14 ligands have been identified, but the affinity of none of them was determined. Modifications were introduced in position 5 or 9 of the sialic acid. The binding to Siglec-5 or Siglec-5/Siglec-14 was evaluated in two ways: by glycan microarray or by metabolic incorporation in cells. In this last case, the ligands underwent further derivatization by click chemistry; the binding was analyzed by flow cytometry. (Büll et al., 2017; Rillahan et al., 2012)