MAG (Siglec-4) is the most conserved among Siglecs and, unlike the rest, does not have any involvement in the immune system. Indeed, it occurs on oligodendrocytes and Schwann cells in the central and peripheral nervous systems. (Lopez, 2014) It is a highly glycosylated protein, presenting one V-set domain and four C2-set domains in the extracellular region and, as Sialoadhesin, none ITIM or ITAM intracellular motif. (Quarles, 2007) The cytoplasmatic tail can be of two different lengths leading to two isoforms, the L-MAG and S-MAG. The L-MAG presents a Fyn-kinase phosphorylation site.(Lai et al., 1987; Yamauchi et al., 2012) By interacting (trans-interactions) with neuronal gangliosides such as GT1b and GD1a, MAG assures the correct spacing between the axon and the myelin. Controversial results have been obtained regarding MAG’s biological function, but different studies support the theory that it inhibits axonal regeneration. Upon myelin disruption, MAG can also diffuse as a soluble form to other sites and significantly inhibit axon growth. IThis explains why neurons degenerate even in the absence of myelin disruption in the early phases of multiple sclerosis. On the other hand, MAG intervenes in the regulation of myelination, mainly via the activation of the Fyn-kinase. The mechanism behind its functions is not entirely elucidated yet. A study showed how the trans-interaction could explain the bi-directional signaling with the ganglioside and the cis homodimerization observed through the domains Ig4 and Ig5. (Pronker et al., 2016) Like other Siglecs, MAG is an endocytic receptor: via a clathrin-dependent mechanism, it is recycled in the membrane contributing to its modeling. (Winterstein et al., 2008)
Different strategies have been pursued to understand its function and roles better. An anti-MAG humanized monoclonal antibody (mAb) from Glaxo (GSK24932) underwent clinical trials for promoting neuronal regenerations in strokes, blocking MAG inhibitory activity. (Barbay et al., 2015; McKerracher & Rosen, 2015) Unfortunately, the positive results observed in squirrel monkeys did not translate in humans: however, there was no direct evidence that the therapy reached the target, so further studies are necessary. (Cramer et al., 2017) Different efforts focused on developing small molecules. (Schwardt et al., 2015) The natural ligands of MAG are α2-3 sialosides which bind to the target protein in the low micromolar range. They are a good starting point compared to other Siglecs, which bind their natural ligands with less affinity.
The full extracellular portion of MAG has been crystallized. The co-crystallization of MAG with 3’-N-Acetylneuraminyl-N-acetyllactosamine helped understand the main interactions with the ligands (Figure 9). As for other Siglecs, the conservative Arg118 forms a salt bridge with the carboxylate of the sialic acid. A hydrogen bonds network involves the hydroxyl group in positions 4, 8, 9 and the NH in 5 with the surrounding Tyr65, Thr128, Tyr124, and Asn126. Position 9 of the sialic acid is close to a hydrophobic pocket formed by the sidechains of Pro64 and Tyr65. (Pronker et al., 2016)
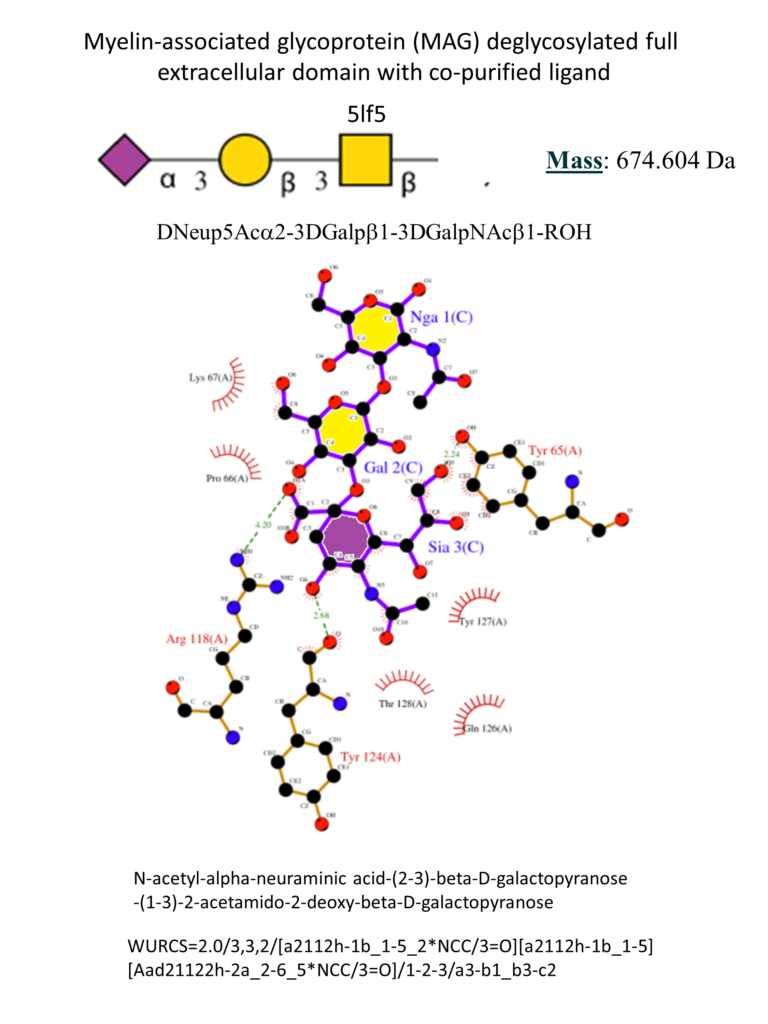
Small molecules have been synthesized to target MAG and possibly better understand its biological function. (Schwardt et al., 2015) In the B. Ernst group, starting from the minimal binding epitope Neu5Ac-α(2-3)-Gal-β(1-3)-GalNAc, contained in the natural gangliosides GD1a and GT1b, different strategies contributed to identifying high-affinity MAG antagonists (Figure 10).
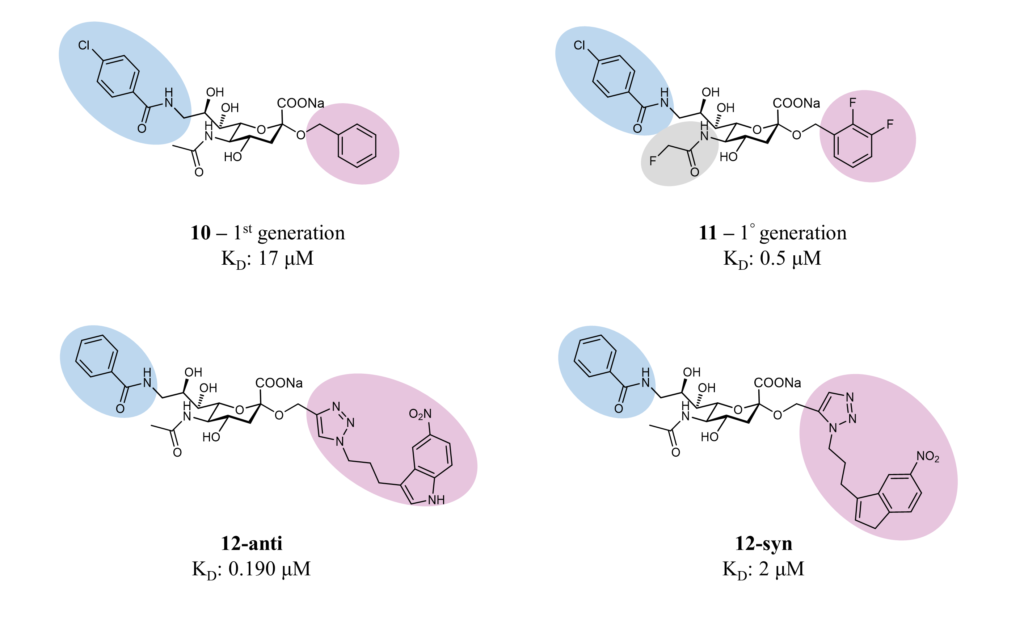
In the first two generations of ligands, different modifications were introduced in positions 2, 5 and 9: in particular, a difluorobenzyl substituent at the 2-position, fluoroacetate at the 5-position, and a p-chlorobenzamide at the 9-position (compounds 10 and 11, Figure 10). These beneficial modifications led to a ligand with nanomolar affinity and drug-like properties.(Mesch et al., 2010; Shelke et al., 2007) Later, a combinatorial fragment-based drug discovery strategy identified fragments that could fit into the binding pocket around the sialic acid. The screening identified the 5-nitroindole group, which was subsequently attached to position 9 by click chemistry, leading to high-affinity ligands 12-syn (2 μM) and 12-anti (0.19 μM), as determined by surface plasmon resonance (SPR). (Shelke et al., 2010)
