Siglec-9 occurs in human blood on neutrophils, monocytes, plasma cells and a subset of NK cells. It is an inhibitory protein, thus presenting in the cytoplasmatic tail the two putative ITIM domains, while on the extracellular portion, the standard V-set domain and two C-2 set domains. (Zhang et al., 2000)
Considering its expression and the inhibitory activity on cells of the immune system, Siglec-9 represents a potential target for cancer, inflammations and viral infections.
Malignant cells usually have a different glycosylation pattern, resulting in hyper sialylated glycans, therefore providing a high number of ligands for Siglecs. (Büll et al., 2014; Fuster & Esko, 2005) T-cells and NK cells are both tumour-infiltrating cells, participating in the recognition and killing of cancer cells, and they both express Siglec-7 and Siglec-9. (Daly et al., 2019) Siglec-9 is upregulated in some tumour-infiltrating CD8+ and CD4+ T-cells in various cancers. Studies show that the binding of Siglec-9 with antigen-binding fragments (Fab) of Siglec-9-blocking antibodies increased immune cell activity, reducing the interactions of Siglecs with their ligands. The same effect was reached by treating target cancer cells with neuraminidases, which decrease the availability of sialic acid on the surface and, consequently, the interactions with Siglecs, thus enhancing the immune response. However, the treatment with full-length anti-Siglec antibodies inhibits cell cytotoxicity, suggesting the induction of inhibitory signals.(Haas et al., 2019; Hudak et al., 2014; Jandus et al., 2014; Nicoll et al., 2003; Stanczak et al., 2018) A better comprehension of Siglec-9 signal regulation is therefore needed.
A recent study shows how blocking Siglec-9 interactions with sialylated sugars on NK cells increased their cytotoxicity toward HIV-infected cells. (Adeniji et al., 2021)
Another trick that pathogens use to escape the immune system is to mimic self sialic acid-containing sugars. In this way, Group B Streptococcus (GBP) binds to Siglec-9 expressed on neutrophils. As an inhibitory receptor, this leads to the deactivation of the immune response and the bactericidal activity. (Chang & Nizet, 2020) Siglec-9 can also be a target for treating other diseases associated with neutrophils, such as asthma, chronic obstructive pulmonary disease, and pulmonary disorders related to neutrophilia. Recent evidence also suggests a role of neutrophils in the inflammation due to Covid infection. The binding and clustering of Siglec-9 on the cell surface of neutrophils with monoclonal antibodies or polyvalent sialoglycan ligands induce death in neutrophils, significantly when activated. (Chen et al., 2018; Delaveris et al., 2021; Von Gunten et al., 2005)
Siglec-9 shows high sequence homology with Siglec-7, but despite this, Siglec-9 prefers the binding to α2-6 and α2-3-sialyl residues, while Siglec-7 prefers α2-8 linkages. (Varki & Angata, 2006) In particular, a glycan microarray showed a preference for sialyl Lewisx, or the same tetrasaccharide with a sulfate group on the GlcNac moiety. (Yu et al., 2017)
Up to date, the Siglec-9 structure remains unknown.
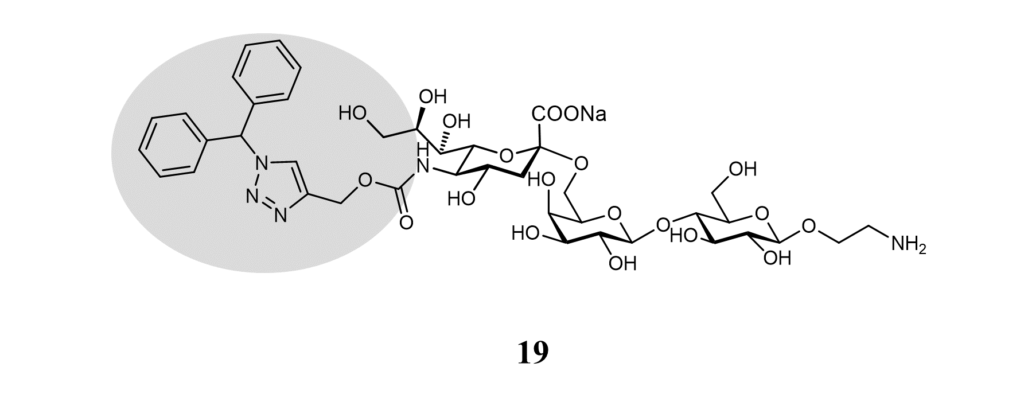
As for other Siglecs, glycan micro-arrays lead to identifying a selective Siglec-9 ligand (Figure 16). No affinity was measured, but liposomes displaying ligand 19 selectively targeted Siglec-9+ peripheral blood mononuclear cells. (Rillahan et al., 2012)