Siglec-8 was discovered around 2000 by two groups independently and identified as a Siglec protein exclusively expressed on eosinophils and mast cells and weakly on basophils.(Floyd et al., 2000; Kikly et al., 2000) Siglec-8 consists of a V-set domain, two C-2 set domains, one ITIM and one ITIM-like domain in the intracellular region. It is involved in negative cell signaling. Siglec-8 cross-linking induces apoptosis in eosinophils, in a caspase-dependent manner on normal cells, and through the production of reactive oxygen species (ROS) in interleukin-5 (IL-5) primed eosinophils. (Nutku-bilir et al., 2008; Nutku et al., 2003) On mast cells, Siglec-8 inhibits their degranulation, but it does not affect their survival. (Yokoi et al., 2008)
Due to the selectivity of expression, the inhibition activity that Siglec-8 has on eosinophils and mast cells, and the constant levels of expression on cells both in healthy and non-healthy conditions, in the last years, much interest has emerged to target this protein for the treatment of many allergic and inflammatory diseases associated with eosinophils and mast cells.
Different results have already validated its potential as a target. AK002 (lirentelimab), for example, is a humanized non-fucosylated IgG1 anti-Siglec-8 antibody already in phase I clinical trials for the treatment of indolent systemic mastocytosis (ISM) and allergic conjunctivitis. The drug improved patients’ general quality of life and relieved comorbidities like asthma, dermatitis, and rhinitis.(Levine et al., 2020; Siebenhaar et al., 2019) Encouraging results were reported for phase II trials to treat chronic refractory urticaria (on patients not responding to antihistamines or omalizumab) and eosinophilic gastritis and duodenitis. Long-term use was well tolerated in the last case and showed histological improvements.(Altrichter et al., 2019; Dellon et al., 2020)
Anti-Siglec-8 monoclonal antibodies reduced non-allergic inflammation by inhibiting IgE-independent mast cell activation. (Schanin et al., 2021)
Several findings also support the idea of using Siglec-8 in the treatment of asthma. For example, there is an upregulation of Siglec-8 ligands in inflamed human airway tissues compared to healthy tissues, and eosinophils obtained from allergy patients showed increased Siglec-8-mediated apoptosis.(Jia et al., 2015; von Gunten et al., 2007) In addition, polymorphisms in the Siglec-8 gene are associated with increased susceptibility to asthma. (Gao et al., 2010)
Lastly, it seems that eosinophils and mast cells are also quite active during SARS-CoV-2 infections and that treatment with anti-Siglec-8 antibodies reduces the general inflammation. Hence, upon confirmation by further studies, Siglec-8 may represent a possible target to fight this viral infection causing the actual global pandemic. (Gebremeskel et al., 2021)
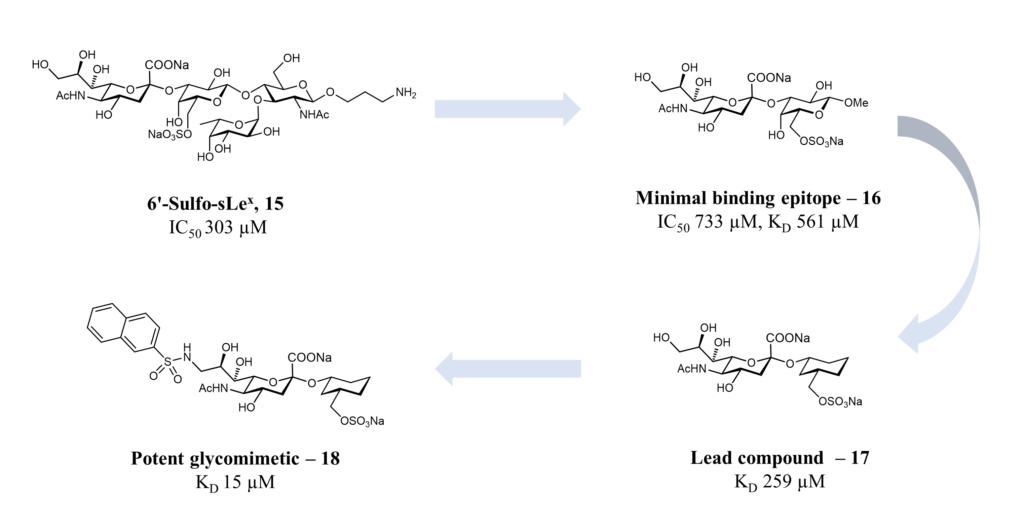
The natural ligand of Siglec-8 is still unknown. In a glycan array screening, the tetrasaccharide 6’-sulfo-sLex (NeuAcα2-3[6-O-sulfo]Galβ1-4[Fucα1-3]GlcNAc) 15 was identified as the preferred Siglec-8 ligand (Figure 15). From this screening, it became evident that Siglec-8 is very selective for α2-3 linkages. The sulfate in position 6 of the galactose seems detrimental to the binding (28-fold loss of affinity when removed).(Bochner et al., 2005 ; Pröpster et al., 2016) Later, an NMR solution structure disclosed the Siglec-8 carbohydrate-binding domain (CRD) conformation in complex with this tetrasaccharide (Figure 14). (Pröpster et al., 2016)
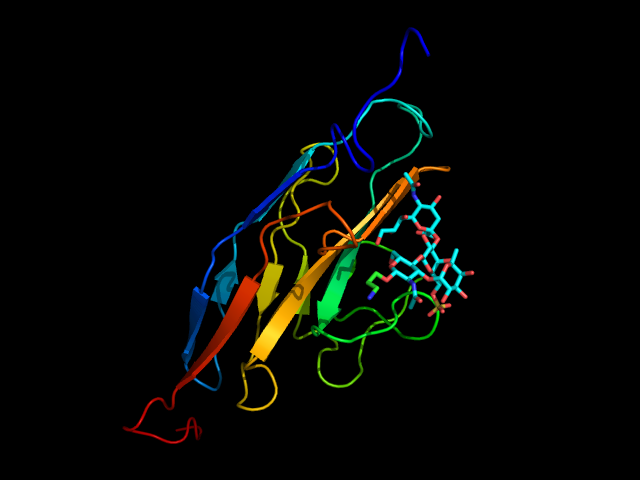
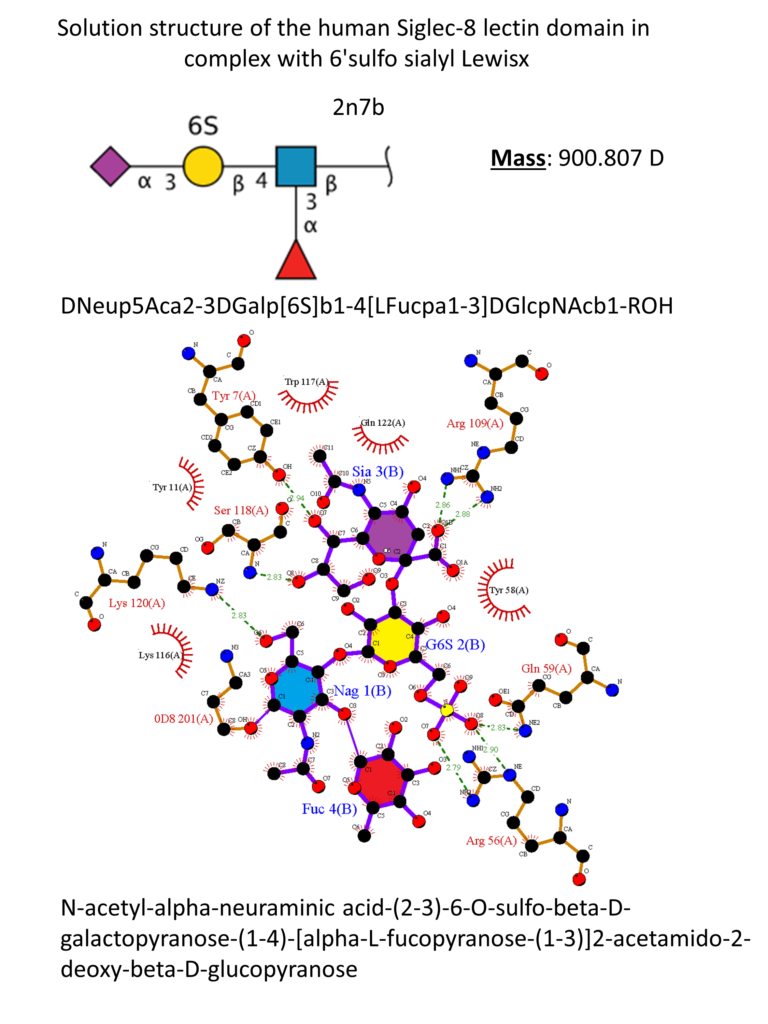
As for the other Siglecs, the main interaction involves a salt bridge between the carboxylate of the sialic acid and the conserved arginine residue (Arg109). The essential sulfate in position 6 of the galactose moiety is involved in a second salt bridge with Arg56 and Gln59. In addition, hydrogen bonds exist between hydroxyl groups 7, 8 and 9 of the sialic acid and Tyr7, Ser118 and Gln122. (Pröpster et al., 2016) Considering that the fucose and glucosamine subunits show only minor interactions with the protein, the 6-sulfo-Sia-Gal 16 is likely to be the minimal binding epitope for Siglec-8. Further optimization with a deoxygenation strategy and introducing a sulfonamide substituent in position 9 of the sialic acid led to identifying a potent Siglec-8 ligand (18), with 20-fold affinity improvement compared to the parent tetrasaccharide (Figure 15). (Kroezen et al., 2020; Nycholat et al., 2019)
The selective expression of Siglec-8 and endocytic property can also be exploited for the selective delivery of therapeutic agents to mast cells and eosinophils to treat malignancies associated with these cells. (O’Sullivan et al., 2018) Next to antibodies, Siglec-8 has also been targeted with nanoparticles displaying its ligands. Liposomes decorated with Siglec-8 ligands were selectively taken up in cells expressing Siglec-8 or Siglec-F (Siglec-8 paralog in mouse). Furthermore, these liposomes could suppress IgE-mediated mast cell degranulation when additionally decorated with allergens.(Duan et al., 2021; Nycholat et al., 2019)
