Siglec-15 is a high evolutionary conserved Siglec, characterized in 2007. (Angata et al., 2007) It exclusively occurs on myeloid cells and osteoclasts. Structurally, it only presents one V-set domain and one C2-set domain, while in the intracellular region is coupled, through the positively charged Lys274, with the activating co-receptors DAP12 and DAP10. Unlike the other activating Siglecs, it does not have an inhibitory counterpart.
Siglec-15 stimulates bone absorption and osteoclasts differentiation on osteoclasts, as confirmed by Siglec-15 targeting with monoclonal antibodies. These findings suggest that targeting this protein could be a successful therapeutic strategy for treating diseases associated with bone loss, like osteoporosis. The use of an anti-Siglec-15 antibody in mice showed potent osteoclastogenesis inhibition, bone resorption suppression, and an overall increase of bone strength. (Tsuda et al., 2022) Other studies showed how a Siglec-15-neutralizing antibody also stimulates bone formation in estrogen deficiency-induced osteoporosis and promotes ossification at cortical bone’s damaged area in fracture healing mouse models. (Zhen et al., 2021)
Siglec-15 also plays a role in the tumor microenvironment and it is expressed on tumour-associated macrophages. Some results showed that Siglec-15 suppresses T cell proliferation, essential in regulating tumor growth. (Wang et al., 2019) Cancer growth upon targeting Siglec-15 with a monoclonal antibody in wild-type mice was limited: in this disease model, it seems that Siglec-15 acts as a ligand for an unknown receptor expressed on T cells. Whether the interactions with sialic acids and Siglec-15 are involved in this process is still unknown. (Angata, 2020) A humanized anti-Siglec-15 mAb, the NC318, will soon start the phase II clinical trial to treat advanced solid tumors. In phase I, it was well tolerated, and it showed prolonged stabilization of the disease. (Angata, 2020)
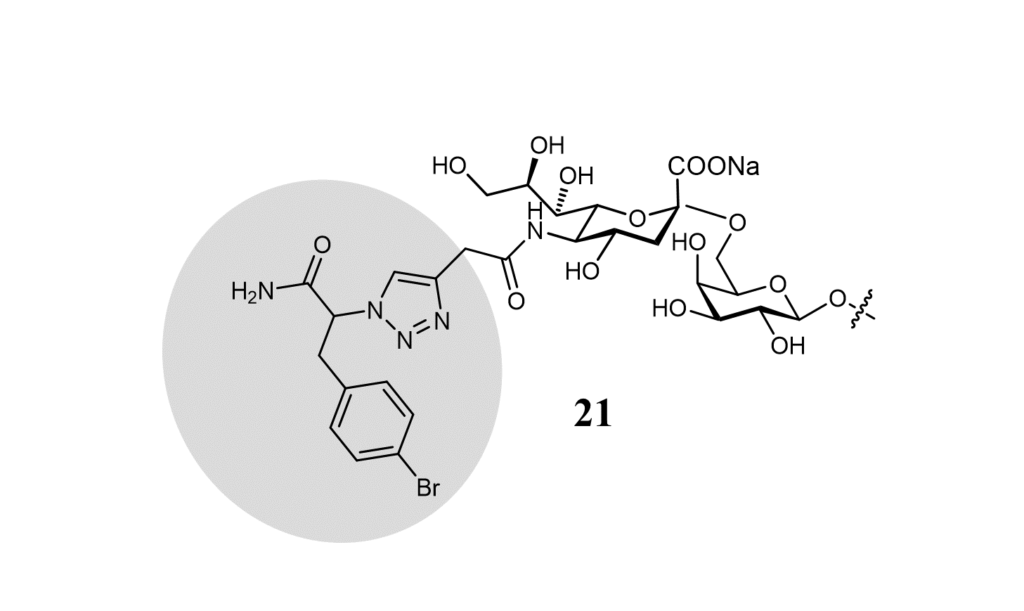
The preferred ligand of Siglec-15 is the Sialyl-Tn (Neu5Acα2-6GalNAc). (Angata et al., 2007) A cell-based glycan array helped identify other possible ligands. Cells lacking sialic acids on the surface were functionalized by introducing alkyne-modified sialic acid on cell surface glycoconjugates by sialyltransferase. Click chemistry reactions were then employed to derivatize the alkyne group with different types of azides. Siglec-15 binds to α2-6-sialosides but also α2-6-linked sialosides. (Briard et al., 2018) Ligand 21 shown in the figure below, was identified as the best ligand of this series (Figure 18).
Future studies that may better reveal the mechanism involved in Siglec-15/T-cells interactions and the identification of potent inhibitors could open the way to new therapeutic options for cancer and diseases associated with bone loss.
