CD33 (Siglec-3) is one of the first Siglecs discovered, and it is the shortest member of the Siglecs family. The extracellular portion presents a V-set domain, which mediates the interaction with the sialic acid, and one C-2 Ig domain: because of its short length, it is more prone to bind ligands on the same cell surface. (Freeman et al., 1995) Alternative splicing of CD33 RNA leads to a shorter isoform that lacks the V-set domain. (Pérez-Oliva et al., 2011) It exhibits ITIM and ITIM-like domains on the cytosolic tail; the first one is absent in mouse. As mentioned in the previous sections, these domains are involved in inhibitory signaling with the typical cascade common to other Siglecs. (Paul et al., 2000) Next to SH2-phosphatase, ITIM can also be bound by the suppressor of cytokine signaling 3 (SOCS3). It leads to ubiquitination and consequent degradation of CD33: this mechanism causes the loss of this receptor during an inflammatory process. (Orr et al., 2007) CD33 internalizes upon binding or dimerization, particularly with antibodies: this process decreases the overall general availability of CD33 on the cell surface. (Walter et al., 2008)
CD33 is expressed on myeloid progenitors, macrophages, monocytes, microglia and granulocytes, regulating phagocytosis and inflammatory responses. (Bhattacherjee et al., 2019; Crocker et al., 2012; Macauley et al., 2014) In particular, CD33 has been identified as a marker and target for acute myeloid leukemia (AML) since it is highly expressed on AML blasts. (Ehninger et al., 2014; Robertson et al., 1992) Gentuzumab ozogamicin is a CD33antibody-drug conjugate approved by the FDA for the treatment of AML: upon internalization, it releases calicheamicin, a drug able to bind the DNA and cause cell death. (Maakaron et al., 2021)
Dysregulation of microglial phagocytic activity is also connected to Alzheimer’s disease (AD), with reduced ability to remove amyloid-beta (Aβ) plaques in the brain. Pieces of evidence show that polymorphisms in the CD33 gene may represent a risk for AD, which would also affect microglia phagocytic activity. However, these studies have been conducted on mice, which present a different form of CD33. Therefore, these conclusions remain to be confirmed. A recently presented human CD33 knock-in mouse model might answer these open questions. (Griciuc et al., 2013) Besides, an anti-CD33 antibody is in phase I clinical trials to treat AD.
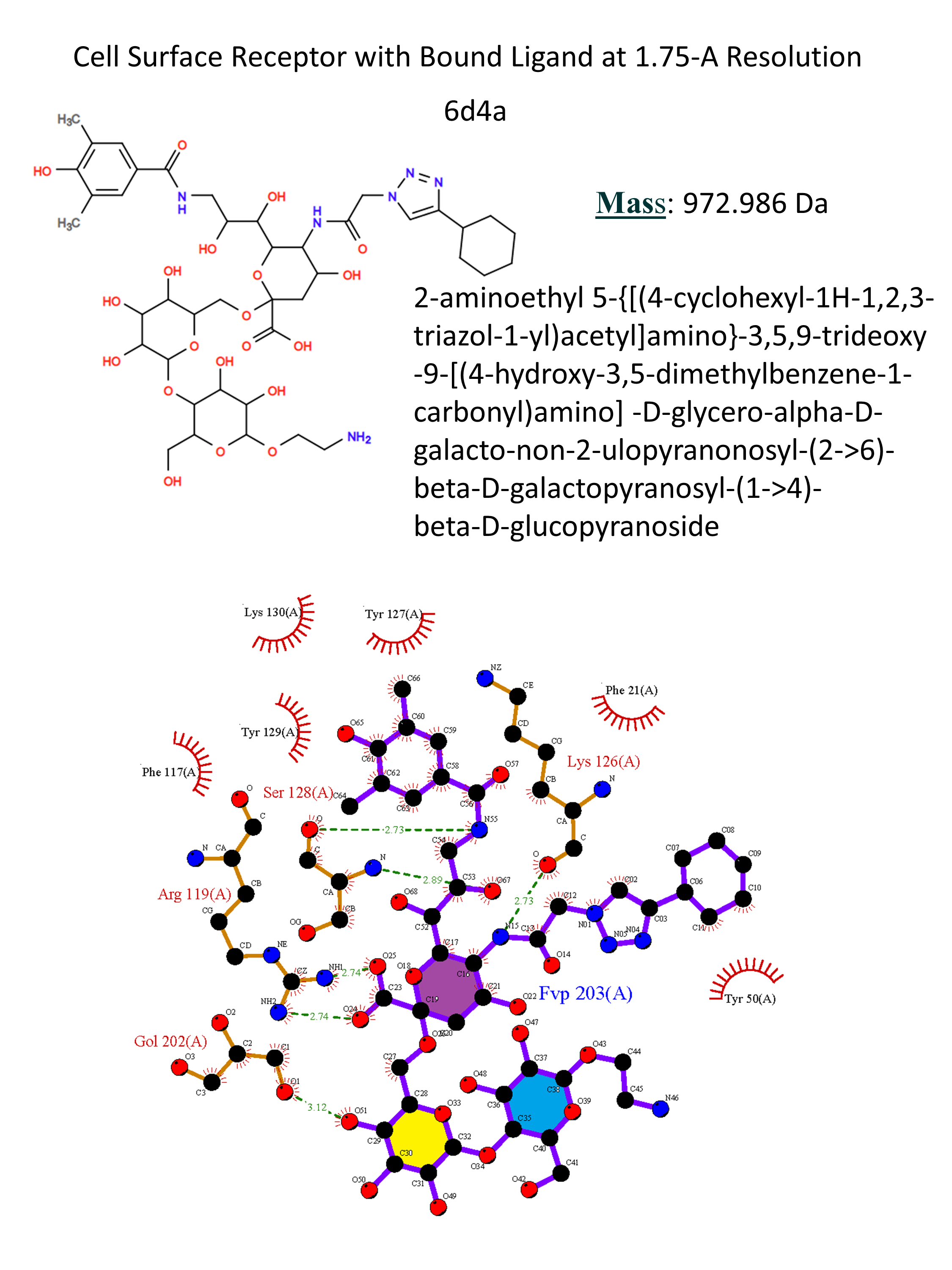
As for the other Siglecs, the conservative Arg119 forms a salt bridge with the sialic acid. Around it, two clefts accommodate the hydrophobic substituents in positions 5 and 9. (Movsisyan & Macauley, 2020) Presentation of ligand 9 on microparticles to microglial cells increased the uptake of the toxic peptide amyloid-β involved in the AD. (Miles et al., 2019) Besides, the interaction between liposomes presenting allergen and ligand 9 and mast cells suppressed IgE-mediated mast cells’ activation. (Duan et al., 2019) These two recent findings confirm how CD33 represents a very interesting pharmacological target for AD treatment and preventing allergic reactions.
