CD22 (Siglec-2) is one of the best described Siglecs. It is well conserved across mammals, and it is highly expressed on B cells, where it regulates their function.
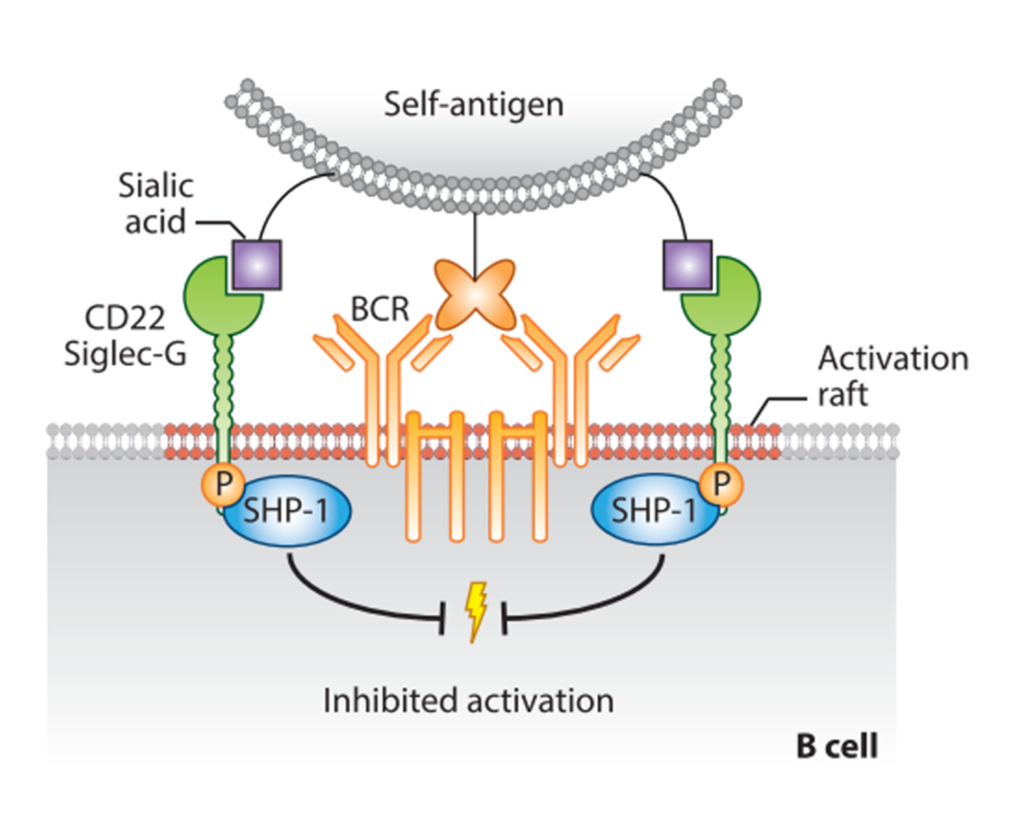
There are seven Ig-like domains in the extracellular region, where the V-set domain contains the sialic acid-binding site, as in every Siglecs. The cytoplasmatic tail contains five domains, three ITIM, one ITIM-like, and one Grb2 binding motif, involved in the negative signaling. Crocker et al., 2007) CD22 is indeed an inhibitory co-receptor of the B cell receptor (BCR), and it helps set a threshold for the activation of the cell itself. (Tsubata, 2012) To suppress the BCR signals, CD22 should be in its proximity: this will result in phosphorylation of the ITIMs by the SRC-family kinase LYN, the recruitment of SHP1, the dephosphorylation of BCR complex and the following downregulation of the signal (Figure 5).(Nitschke, 2005; Tedder et al., 2005) In the resting state, CD22, sialylated, is usually “masked” by cis-interactions with ligands expressed on adjacent CD22 molecules, forming clusters and limiting its association with the BCR. Despite this, CD22 masking does not entirely prevent trans-interactions and the distribution to cell-cell contacts sites where the BCR is also involved.(Collins et al., 2004; Enterina et al., 2019; Poe et al., 2004)
Considering its inhibitory activity, CD22 is a potential target for treating B cells-associated malignancies or autoimmune disorders, such as hairy cell leukemia, chronic lymphocytic leukemia, and non-Hodgkin lymphoma.(Clark & Giltiay, 2018; Dörner et al., 2012; Sullivan-Chang et al., 2013a)
One of the strategies exploited to target CD22 is the use of antibodies. (Angata et al., 2015) The first fully humanized anti-CD22 IgG1antibody is Epratuzamab (Emab): it does not kill malignant B cells as a single agent, but it showed positive results when associated with Rituximab. (Leonard & Goldenbergm, 2007; Sullivan-Chang et al., 2013b) Clinical trials have also been conducted on the use of Emab for the treatment of autoimmune diseases like systemic lupus erythematosus (SLE).(Geh & Gordon, 2018; Gottenberg et al., 2018) Since upon antibody (Ab) binding CD22 is internalized, another approach is using Ab-conjugated drugs, such as inotuzumab ozogamicin, where the Ab is conjugated with a drug able to disrupt the DNA. This agent is approved for treating acute lymphoblastic leukemia (ALL). (Kantarjian et al., 2016) Recently, CD22 has been a target for CAR T cell therapies to redirect T cells effectors towards malignant B cells: positive results were obtained for the treatment of B cell ALL. (Adeel et al., 2021)
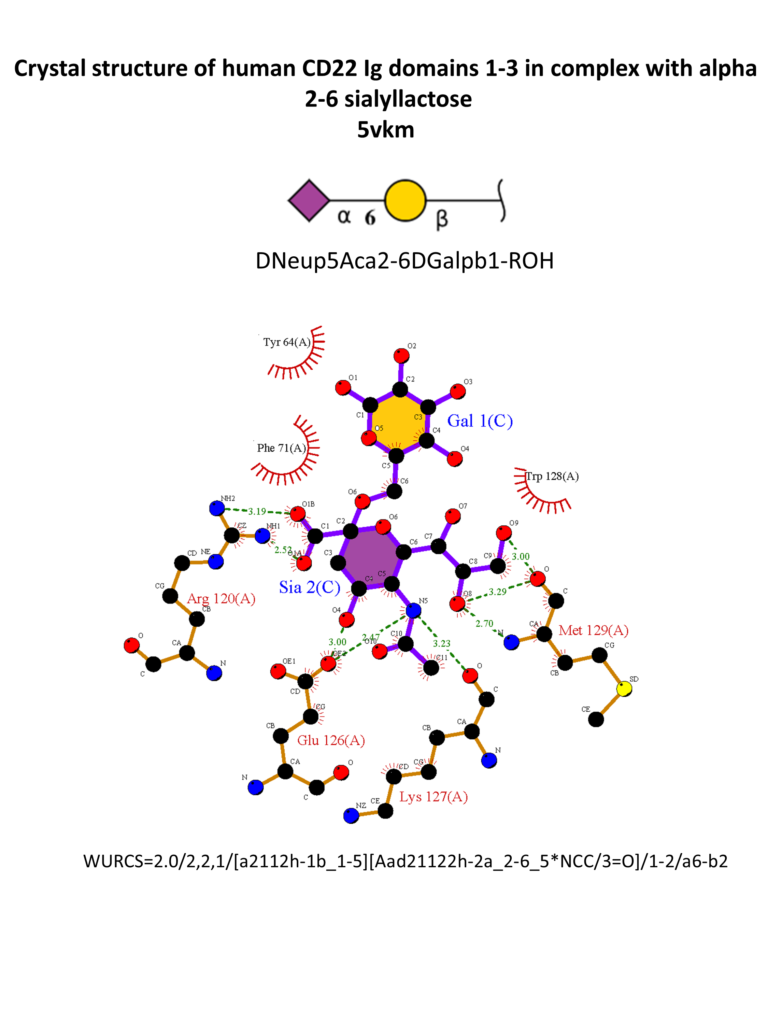
Different strategies were also adopted for the synthesis of high-affinity ligands for CD22. CD22 has a strong preference for α2-6 Sia ligands, in particular Neu5Acα2-6Galβ1-4GlcNAc (Neu5Acα2-6LacNAc), with an affinity range between 50-200 μM, and α2-6-sialylated 6-sulfo-LacNac.(Kimura et al., 2007; Macauley et al., 2015) The 3D structure of CD22 Ig domains 1-3 has been determined (Figure 6). (Ereño-Orbea et al., 2017) The conservative Arg120 is essential; mutations in this position abrogate the binding. (Van Der Merwe et al., 1996) Other important interactions involve the N-acetamido substituent and the glycerol chain of the neuraminic acid, H-bonded with Met129. A hydrophobic pocket close to position 9 of the sialic acid accommodates large and aromatic substituents. α2-3 linked ligands would clash with the Tyr64, so CD22 is specific for α2-6 linkages.(Kelm et al., 1998; Movsisyan & Macauley, 2020)
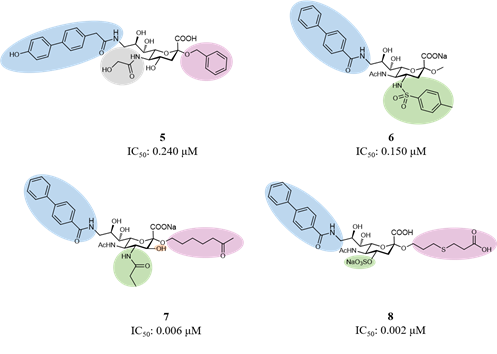
Considering the main interactions, methyl-α-Neu5Ac has been the starting point for initial modifications. As for Sialoadhesin, introducing a biphenyl moiety in position 9 of the sialic acid improved the affinity for CD22. (Kelm et al., 2002) Then, combinations of favorable fragments in different positions of the sialic acid, like positions 2, 4, 5 and 9, led to the discovery of several ligands with high potency and selectivity for CD22 (Figure 7).(Abdu-allah et al., 2011; Abdu-Allah et al., 2020; Kelm et al., 2013; Prescher et al., 2014) Compound 5 was the first monomeric ligand to show high affinity to a Siglec protein, to regulate B cell activation in vitro and the immune response in vivo.(Abdu-allah et al., 2011; Matsubara et al., 2018; Sheikh et al., 2018)
High-affinity monovalent ligands can also target CD22 by multivalent presentations. For example, the binding of CD22 with liposomal nanoparticles covered by both antigens and CD22 ligands causes the apoptosis of the B cell in mice and humans: these Siglec-engaging tolerance-inducing antigenic liposomes (STALs) can be exploited to tolerize B cells to specific antigens and to prevent an unwanted immune response.(Macauley et al., 2013)
The endocytic properties of CD22 are used for delivering cargo to B cells. Indeed, liposomal particles can be internalized, CD22 will be recycled to the membrane surface, and the cargo will stay in the cell. (O’Reilly et al., 2011) This strategy successfully delivered doxorubicin to the B cells, resulting in killing B cells lymphoma both in vitro and in mice. Besides, a soluble dimeric high-affinity CD22 ligand conjugated to a toxin was efficient in killing B cells ALL. (Chen et al., 2010; Schweizer et al., 2012). As an alternative to antibodies or nanoparticles, multivalent ligands conjugated to toxins were efficiently internalized and caused the death of malignant B cells. (Peng & Paulson, 2017)
