Wounds and wound healing process
The skin is an organ protecting the human body from pathogens. A wound is a physical disruption of the skin, and therefore, its protective functions against the environment and pathogen microorganisms are lost. Wounds can be caused by abrasion, laceration, puncture, avulsion or burns. Chronic wounds, in contrast, are caused by a pathological process and include venous ulcers, diabetic and pressure ulcers. Depending on its severity, a wound will affect different depths of the skin: epidermis, superficial dermis, deep dermis and even reach the underlying muscle layer.
In the process of wound healing, four overlapping steps are observed. First, blood leaking is stopped in the hemostasis process. The presence of platelets supports blood clotting and coagulation. The inflammatory phase begins at the same time. Characteristic swelling and redness of the wound is observed and is caused by the presence of white blood cells, growth factors, nutrients and enzymes. White blood cells make the substance called the exudate. Damaged cells and bacteria are removed in the process of phagocytosis. The new tissues are rebuilt in the proliferation phase. Fibroblasts grow, collagen and the extracellular matrix is rebuilt as well as the blood vessels network. Finally, remodelling and closing of the wound occur. This is the maturation phase.
The amount of exudate is a parameter to monitor and control. Depending on the amount of exudate a wound can be dry, moist, wet (when the primary wound dressing is wet), saturated (when free fluid is visible, leakage on the secondary dressing), or leaking (when saturation of the secondary dressing occurs). The primary dressing is in direct contact with the wound while the secondary dressing maintains the primary dressing in place. Some wounds such as burns, inflammatory ulcers and skin donor sites are more likely to present high rates of exudate production that must be controlled. Inflammation phase can be a concern if it lasts an excessive amount of time. In this case, microbial contamination can be suspected. Moreover, dark granulation of tissues in the proliferation phase can be a sign of infection.
The wound is a suitable environment for bacteria establishment and infection development. It provides warm and moist conditions and the presence of nutrients. Burns tend to be the most exposed wounds towards infections as a large surface of skin integrity is lost.
Microbiology of a wound
Non-replicating microorganisms are found in wounds. This is known as wound contamination. When the microorganisms adherent to the wound can replicate, the process is known as wound colonization. A major concern occurs when the replicating microorganisms cause injury to the host and wound infection is established. Bacteria, fungi and viruses can cause wound infections. In 1989, Gristina et al. came up with the expression “race for the surface”, illustrating the process of attachment of microorganisms and biofilm development on implanted biomaterials (Gristina et al. 1989).
From a literature review of clinical papers recording the bacteria present in infected wounds, it clearly appears that seven bacteria are very often detected. They make up around 80 % of the wound microbial flora (Figure I. 21 left). When the bacterial flora is separated as a function of the type of wound, different distribution profiles are observed (Figure 21 right).
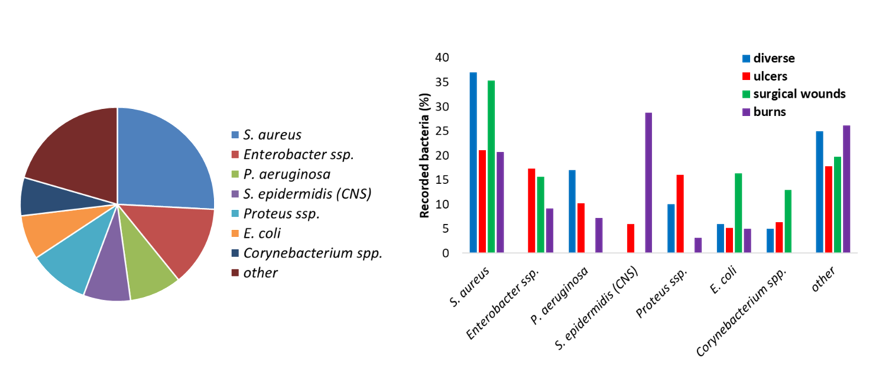
Figure 21: Bacteria found in wound infections (left) and the evolution of the bacterial flora depending on the type of wound (right). From (Madsen et al. 1996; Erol et al. 2004; Gjødsbøl et al. 2006; Guggenheim et al. 2009; Körber et al. 2010; Turtiainen et al. 2014; Bessa et al. 2015; Park et al. 2017).
The microbial flora is dependent on the type of wound (superficial or necrotic) and is likely to change over time. In early acute wound, the local microflora is present and mostly Gram-positive bacteria are present. After a few weeks of inflammation and retarded healing, facultative anaerobe bacteria will colonize the wounds. If the wound deteriorates and reaches the deeper dermis and muscles, anaerobes are found. Polymicrobial infections (more than one pathogen) are likely. From clinical data of the bacterial flora of 74 lesions, Daltrey et al. observed that some microorganisms were commonly associated with necrotic wounds and the presence of dead tissues, while other to superficial lesions (Daltrey et al. 1981). Besides the bacterial population, infected wounds are often colonized with fungi and more specifically, Candida species (Kalan and Grice 2018).
Definition of a wound dressing
Characteristics and properties of wound dressings. Wound dressings are designed to meet various criteria, depending on the wound type and its state (acute, chronic, inflammatory, proliferation, healing, infected, etc.). The dressing provides mechanical and biological protection from the surrounding environment. Additionally, a closed wound is in constant contact with growth factors. To promote wound healing, an ideal wound dressing would promote a moist environment and meanwhile remove excess exudate and allow gaseous exchange. It must be non-cytotoxic to the wound tissue and be free of any contaminants. In some cases, sterile wound dressings are required. Other properties such as comfort, non-adherence to the wound site, cost-effectiveness, long shelf life are looked upon when selecting a suitable dressing.
Historically, linen strips, wool and clay have been used by Mesopotamian and ancient Greeks to protect the wound site. In the late 20th century, cotton gauzes were used to keep the wounds dry. It is only in the mid-1980s that modern wound dressings would fit the requirements for wound healing by providing a moist environment while removing excess exudate. Today various groups of dressing exist and include hydrogels, hydrocolloids, films, foams, meshes etc. Dabiri et al. listed the recommended wound dressings adapted to specific wound types (Dabiri et al. 2016). The more traditional dressings (bandages, gauze) are used in the case of dry and superficial wounds or as secondary dressings. Two types of wound care products are distinguished: non-occlusive dressings (gauze) and semi-occlusive or occlusive dressings that act as a barrier against bacteria.
Bio-based wound dressings.
Most of the modern wound dressings are prepared from synthetic polymers. A literature analysis indicates that almost 90 % of wound dressings are non-biobased (Figure 23). Nylon derivatives have been used to develop flexible and conformable films dressings; however, they do not remove exudate efficiently (Mölnlycke®, Mepitel®). Polyurethane foams have been developed for lower limb wound and chronic dressings (Shah et al.; Ajit Kumar Varma et al. 2006). A review from Gibas and Janik (2010) listed hydrogels prepared from synthetic polymers and used for biomedical applications. Because of the constant and reproducible chemical and physical characteristics of synthetic polymers, the production of wound care materials is performed in a controlled and homogeneous process. However, the need for sustainable, renewable and biodegradable materials calls for the development of bio-based dressing materials.
Bio-based materials are produced from natural monomers and polymers extracted from a microbial, animal or vegetal source. These polymers generally have good biocompatibility and hemocompatibility, degradability and renewability and their cost are relatively low. Additionally, their chemical structure and organization are similar to the extracellular matrix making them excellent candidates for wound dressing development.
Because of their biocompatibility and versatility, biopolymers are used in the development of a new generation of wound dressings. The most widely used biopolymers are collagen, chitosan, chitin, alginate, cellulose and hyaluronan. Their distribution in the development of bio-based wound dressings is presented in Figure 23. Sahana and Rekha discussed the roles of these biopolymers in the healing process (Sahana and Rekha 2018). This includes fibroblasts proliferation and migration, keratinocytes proliferation, moisture retention, exudate removal, anti-inflammatory properties, simulation of monocytes. Examples of the use of these biopolymers in wound dressing applications are given in this section.
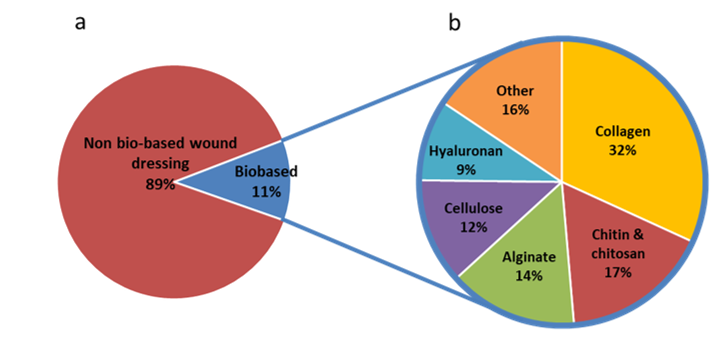
Figure 23: Number of wound dressing publications with the descriptors wound dressing / wound-dressing / wound care and biobased / cellulose / nanocellulose / chitin / chitosan / hyaluron* / collagen / alginate on Scopus July 2019.
Collagen & hyaluronan are both animal-sourced and are present in the extracellular matrix and the skin, respectively. Collagen is a protein found in the form of fibrils. Native and denatured collagen-based dressings have been developed. A review from Brett (2008) discusses the role of collagen as a sacrificial tissue in wound dressing and the control of cellular functions. Collagen enters in the composition of various wound dressings: BioPad®, CellerateRX®, DermaCol®, Fibracol®, Prisma®. These dressings present different structures: gel, matrix, sponge, pads etc. and are adapted for different types of wounds (Fleck and Simman 2011). Collagen is often blended with other biopolymers such as chitosan, alginate (Xie et al. 2018) and cellulose (Jeschke et al. 2005). Hyaluronic acid was used in the composition of skin substitutes and promoted keratinocytes and fibroblast migration (Wang et al. 2006; Niiyama and Kuroyanagi 2014).
Chitin & chitosan . Chitin is mainly biosynthesized by crustaceans and is made of β1,4 N-acetyl glucosamine. Chitosan is obtained from the deacetylation of chitin. When used in the dressing, chitosan was found to promote healing (Stone et al. 2000), exhibited good swelling properties, antibacterial activity and non-toxicity (Xia et al. 2016) as well as increased proliferation of fibroblasts (Mayol et al. 2014). It was introduced in the preparation of cellulose composite membranes to enhance the antimicrobial activity for potential application as a wound dressing (Cao et al. 2016). KytoCel® and Chitoderm® are chitosan wound dressing available as aerogel and chitosan-coated dressing, respectively.
Alginate is extracted from marine algae and are composed of alginic acid made of repeating units of β-D-mannuronic acid and α-L-guluronic acid. Alginate wound dressings were found to reduce pain, absorb exudate while keeping a moist environment. Hydrogel films with high water absorption and swelling behaviour were prepared from alginate solvent casting process (Pereira et al. 2013). They are usually available in the form of fibres, aerogels, hydrogels and hydrocolloids: Algisite®, Algosteril®, NuGel®, Tegagel®, Sorbsan®, Comfeel Plus Flexible®.
Cellulose: One of the advantages of cellulose in wound dressings is its ability to absorb water and therefore, exudate while providing a moist environment at the wound site (Basu et al. 2017). There are some commercially available cellulose-based dressings, such as Dermafill®, Nanoderm® and Vermac®.
Bacterial cellulose , in particular, is used in wound dressings and is considered a suitable material for wound dressings because of its high purity and favourable characteristics of non-toxicity, mechanical stability and high moisture content (Sulaeva et al. 2015). Bacterial cellulose dressings ensure thermal and gaseous exchange while exhibiting permeability to microorganisms. Real-time monitoring of the wound was allowed when transparent bacterial cellulose dressings were produced (Springer et al. 2019). The issue of using bacterial cellulose lies in the high production costs and difficulty of mass production, although becoming less difficult considering the recent developments. On the other hand, large quantities of cellulose nanofibrils and to a lesser extent of cellulose nanocrystals can be produced.
Wound dressings materials from CNFs and CNCs (nanocellulose only or in composites) were also studied. Faster recovery of surgical wounds was observed with CNF dressings in Sun et al. (2017) study. Oxidized-cellulose exhibit good hemostatic properties (Lewis et al. 2013; Vosmanska et al. 2014). Song et al. observed enhanced wound healing for wounds dressed with tunicate CNCs-alginate or tunicate CNCs-selenium membranes (Song et al. 2017). Bacakova et al. reviewed the applications of nanocellulose in tissue engineering and wound healing (Bacakova et al. 2019). Because cellulose does not exhibit antimicrobial activity, it has been functionalized to produce wound dressings with antimicrobial activity (Wiegand et al. 2015b; Napavichayanun et al. 2015). Bioactive wound dressings from biopolymers and more specifically from cellulose are discussed in the next section.
