Nanocellulose present numerous interesting properties of renewability, non-toxicity, good mechanical properties, barrier properties, high specific surface areas etc. Applications areas seem to be endless, from packaging and paper industry to electronics, or even healthcare. To impart new functions to nanocellulose-based materials, the surface modification of CNFs or CNCs is sometimes required. Nanocellulose surface modifications have been widely studied for several applications to counteract the limitations of cellulose nanofibrils, or nanocrystals use in certain conditions (due to their highly hydrophilic nature) and to foster new functions. The presence of hydroxyl groups makes cellulose a highly reactive polymer, and various modifications are available. Many reviews outline the diversity of chemical modifications pathways to modify cellulose nanocrystals (Lin et al. 2012; Eyley and Thielemans 2014; Kedzior et al. 2018) and cellulose nanofibrils (Gandini and Belgacem 2011; Rol et al. 2019) or both (Habibi 2014). Surface modification approaches are usually divided into two groups: grafting (of simple molecules or polymers) and adsorption. In Figure 25, the variety of CNF post-modification strategies is illustrated (Rol et al. 2019).
Physical adsorption is mainly promoted by hydrogen bonding, van der Waals interactions or electrostatic interactions. It is an easy, fast, and usually eco-friendly process. However, the compounds are non-covalently bound to the cellulose, and there is a risk of leaching. TEMPO-oxidation of nanocellulose introduces carboxyl groups on the surface and expand the ionic adsorption or even H-bond interactions. Such adsorptions are sometimes irreversible due to strong interactions and even with low amounts adsorbed the impact on properties can be huge due to the high specific area of nanocellulose. For example, adsorption of a thermoresponsive polyelectrolyte can modify the suspension rheological properties as recently shown by Gicquel et al. (2019). Despite the increasing interest in physicochemical interactions and adsorption, surface modification of nanocellulose has historically been achieved by chemical covalent grafting of molecules or polymers (by “grafting onto” or “grafting from” strategies). Indeed, the hydroxyl groups of the cellulose can react through esterification, acetylation, silylation or carbanylation reactions. Multi-steps strategies were also proposed, such as amidation on oxidized cellulose, for example. The advantage is to have a covalent non-reversible bonding without any leaching of molecules. The drawback is the classic use of toxic solvents. In the following literature review section, the distinction between the modification strategies in organic solvents and green solvents will be made. Indeed, to be consistent with the importance of using renewable materials, their modifications should be done accordingly by respecting the green chemistry concepts. The use of non-hazardous solvents, reduced energy consumption, the reduction of by-products or auxiliary substances and waste management are essential to reduce the environmental impact of the functionalization of nanocellulose materials. For these reasons, new solutions with apolar or hydrophobic reagents, in a green and sustainable approach, will be reported.
Classically, the functionalization of nanocellulose needs apolar organic solvents. Indeed, any reagent which will react with the hydroxyl groups of cellulose can also react with polar solvents like alcohol or water. The traditional organic solvents are usually toxic but allow the use of very reactive reagents like anhydride chlorides or isocyanates, but also the use of strong catalysts for esterification with carboxylic acids, for example. Some examples of possible functionalization reactions are given in the following sections.
Acetylation/esterification.
CNCs have been esterified with various carboxylic acids and special catalysts like tosyl chloride. As an example, valeric acid was grafted to enhance the compatibility of CNCs with poly(lactic acid) by decreasing their hydrophilicity (Shojaeiarani et al. 2019). Cellulose nanofibrils were also esterified with various types of acyl chloride like octadecanoyl and dodecanoyl chloride, in the presence of pyridine, under reflux in toluene (Pasquini et al. 2008). Previously, CNFs were acetylated with acetic acid in N, N-dimethylformamide (DMF) and the presence of pyridine before being incorporated into poly(lactic acid) (PLA) composites (Tingaut et al. 2010). The different esterification procedures of nanocellulose have been recently reviewed by Wang et al. (2018). Aqueous CNC and CNF suspensions were solvent-exchanged to toluene and were esterified with lauroyl chloride in the presence of pyridine (Cunha et al. 2014). After thorough washing steps using different organic solvents, esterified nanocellulose was used as a stabilizer for Pickering emulsions. Indeed, most of the time, an excess of reagents is used, and cautious washing steps are necessary usually using other classic solvents. Acetylation of CNFs with acetic anhydride was performed after a successive solvent exchange of the nanocellulose to acetone and toluene by Mashkour et al. (2015) to improve the barrier and mechanical properties of nanopapers. Propionic anhydride was used to replace acetic anhydride in similar reaction conditions (Singh et al. 2016). Toluene has been widely reported for the esterification of nanocellulose (Rodionova et al. 2011; Mashkour et al. 2015; Singh et al. 2016).
Silylation . Silylation of CNFs with isopropyl dimethylchlorosilane has also been performed in toluene, for example by Goussé et al. (2004) for up to 16 hours at room temperature. Imidazole was used to trap the HCl released. Hexamethyldisilazane has also been used to graft trimethylsilyl groups onto the surface of CNFs in toluene of DMA at 80°C overnight (Johansson et al. 2011). In a fundamental approach, they controlled the reactivity of the hydroxyl groups depending on the solvent.
Isocyanate grafting / carbanylation . CNFs and CNCs were grafted with n-octadecyl isocyanate following two different methods, both involving toluene by Siqueira et al. (2010a). In situ solvent exchange led to the formation of homogeneous grafting compared to solvent exchange before the reaction. This modification technique was also used to enhance the dispersibility of CNCs into PLA composite (Espino-Pérez et al. 2013). Similarly, Missoum et al. grafted an octadecyl isocyanate onto CNFs using an in situ solvent exchange from acetone to toluene procedure. Isocyanate grafting was also performed for fluorescence labelling (Missoum et al. 2014a, b). Amine modified CNFs were prepared by grafting of 4-(Boc-aminomethyl)phenyl isothiocyanate in dimethylsulfoxide (DMSO) (Navarro and Bergström 2014). The CNFs were able to react with carboxylic acid-containing molecules and were labelled with a rhodamine B dye for luminescent CNF images acquisition.
Polymer grafting Nanocellulose can also be modified by polymer grafting and two strategies are reported: “grafting from” and “grafting onto”. Most of these reactions require at least one step with the use of organic solvents. In the “grafting from” strategy, nanocellulose are first reacted with initiator molecules to yield initiator surface-grafted nanocellulose, which are then mixed with monomers to induce polymerization from the surface of nanocellulose. Stenstad et al. grafted for the first time from the CNF surface, a polymer of glycidylmethacrylate (Stenstad et al. 2008). Navarro et al. prepared graft block copolymer modified CNFs by radical polymerization of methyl acrylate and acrylic acid N-hydroxysuccinimide ester (Navarro et al. 2016). Radical polymerization was also used by Zhang et al. (2016) to graft CNFs with poly-isopropyl acrylamide (PNIPAM). Lönnberg et al. grafted polycaprolactone of CNFs in a ring-opening polymerization (Lönnberg et al. 2011). Lönnberg et al. and Tian et al. also used ring-opening polymerization to achieve polycaprolactone grafted CNFs (Lönnberg et al. 2011; Tian et al. 2016). Polymers were also grafted from CNCs with free radical and controlled radical polymerization and ring-opening polymerization. The various studies on polymer grafting from CNCs can be found in Kedzior et al. (2018) recent review.
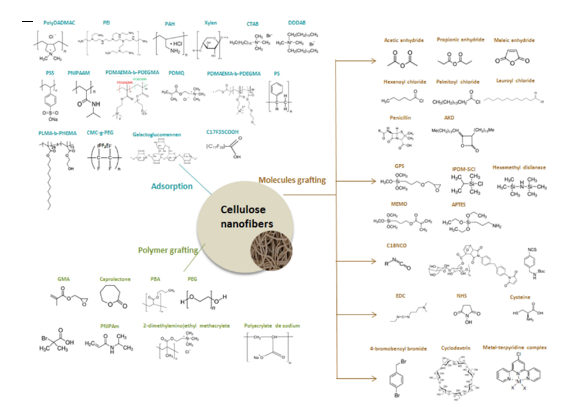
Figure 25: Cellulose nanofibril modifications overview (Rol et al. 2019)
Nanocellulose can also be mixed with a polymer and a coupling agent to promote “grafting onto”. Mulyadi and Deng grafted maleated styrene block copolymers on the surface of cellulose nanofibrils (Mulyadi and Deng 2016). Esterification (Benkaddour et al. 2013), silylation (Yeo and Hwang 2015) or amidation (Barazzouk and Daneault 2012) reactions were also used to graft polymers onto CNF surfaces. Polymers were also grafted onto CNCs by esterification (Ljungberg et al. 2005), isocyanate coupling (Habibi and Dufresne 2008 and Mano et al. 2017) or carbodiimide coupling (Harrisson et al. 2011) in organic solvents such as toluene of DMF.
