In the context of nanocellulose functionalization, the trend goes to greener processes to keep the interest of such sustainable materials. The development of safe and non-toxic modification strategies is essential to provide sustainable materials. The use of bio-based nanocellulose materials is by itself following the green chemistry principles. Green or sustainable chemistry implies the use of clean and non-hazardous chemical processes. It is based on 12 fundamentals principles by Anastas and Warner (1998):
– Prevention of waste rather than cleaning or treating
– Atom economy, also aiming at reducing waste products
– Less hazardous chemical synthesis (or low toxicity) to humans and the environment
– Safer chemicals design
– Safe or reduced use of solvents and auxiliaries
– Design for energy efficiency for minimization of energy consumption
– Use of renewable feedstock
– Reduction of derivatives and additional unnecessary steps
– Use of catalytic reagents
– Design for degradation
– Real-time monitoring for pollution prevention
– Safer chemistry for accident prevention
The solvents used for chemical modification often account for up to 80 % of the total mass. They are usually over-processed (heated, distilled, cooled, filtered, etc.) and result in high-energy consumption. They present risks of toxicity, volatility, inflammability. Because of their great impact on the overall environmental burden, they should be chosen in agreement with a green chemistry approach.
Click Chemistry
Click chemistry meets the requirements of green reactions, by joining two molecules in a fast and efficient way, yielding no or inoffensive by-products and in which the product is easily isolated. The use of the multi-steps approach is possible in a one-pot manner with click chemistry. There is an emerging interest of click chemistry for the functionalization of cellulose substrates. TO-CNFs were functionalized with a metronidazole drug by amidation followed by thiol-ene click reaction for antibacterial applications in Durand (2019) thesis manuscript. A thiol-ene reaction was also performed onto trichlorivinylsilanes modified CNF films with perfluoroalkyl thiols by Guo et al. (2016) to yield superhydrophobicity and by Huang et al. (2014) onto CNC films. Azide-alkyne cycloadditions are also widely used click chemistry reaction with nanocellulose substrates. CNCs were previously modified with azide derivatives for one batch and alkyne derivative for the other and click chemistry cycloaddition brought them together to form a regularly packed CNC material (Filpponen and Argyropoulos 2010). CNCs previously functionalized with a propargyl group were functionalized with reduced graphene oxide by clicking (Kabiri and Namazi 2014). Azide-modified cellulose nanofibrils were modified by propargyl amine using azide-alkyne cycloaddition. Although click reactions are preferably performed in benign solvents, the use of an organic solvent such as DMF, THF, acetone etc. are sometimes necessary.
Ionic liquids Ionic liquids are salts in their liquid state, with a melting point below 100 °C. Because they do not produce organic volatile compounds and because they can be recycled, ionic liquids are considered as green solvents. For example, cellulose nanofibrils were solvent exchanged to acetone and then transferred to an ionic liquid containing acetic, butyric, iso-butyric and hexanoic anhydride reagents (Missoum et al. 2012). Efficient grafting of CNFs was achieved without affecting their morphological properties.
Deep eutectic solvent Deep eutectics solvents (DES) are composed of a mixture of compounds with a melting point lower than each compound on its own. There is recent but growing interest in DES for the production and modification of cellulose nanocrystals and cellulose nanofibrils. In a review by Zdanowicz et al. (2018), their use as a solvent for polysaccharides treatments is illustrated. Cationic functionalization in DES was first reported by Abbott et al. (2006). Ammonium-thiocyanate-urea and guanidine hydrochloride-urea DES were used for cellulose fibres disintegration by Li et al. (2017). Anionic nanofibers were obtained by adding succinic anhydride to a DES composed of trimethylammonium chloride and imidazole (Sirviö and Visanko 2017). Deep eutectic solvents are also used for the dissolution of cellulose and the preparation of CNFs and CNCs.
Emulsions Acylation of cellulose is usually performed in organic solvents. Acylation was performed by freeze-drying and heating of an aqueous system (Yuan et al. 2006). Iso-octadecenylsuccinic anhydride (iso-ODSA) and n-tetradecenyl succinic anhydride emulsions were added to the suspension of CNCs. After a filtration step, they were freeze-dried and subsequently heated at 105°C for various amount of time up to 4 hours. Additionally, the presence of alkenyl succinic anhydride prevented hydrogen bonding during the drying and heating processes. Hydrophobicity was imparted to the modified CNCs and improved their dispersibility in organic solvents and compatibility with polystyrene. A nanoemulsion process was developed by InoFib (Missoum et al. 2016) to modify CNFs with alkyl ketene dimer (AKD). This procedure resulted in hydrophobized CNFs used in coatings formulations and improved air permeance and mechanical strength.
Nanocellulose functionalization in water
Nanocellulose functionalization in water presents the interest to avoid any additional solvent exchange step and to provide a safe and environmentally friendly reaction medium.
Physical adsorption The main drawback of water-based reaction is that it strongly limits the type of reagent used. For example, isocyanate or acyl chloride will be “deactivated” and strongly react with the hydroxyl groups of water before reacting with the hydroxyl groups of cellulose. This is why Physico-chemical adsorption is usually preferred in this case. A cationic surfactant (cetyltrimethylammonium bromide) was adsorbed on the surface of negatively charged CNCs (Qing et al. 2016). Surface modification was proved to improve the drug loading of luteolin and luteoloside, compounds with anti-inflammatory and anti-cancer activity. Adsorption of quaternary ammonium salts was performed in aqueous solution at basic pH (Salajková et al. 2012). The hydrophobicity of the modified CNC surfaces increased, and they could be redispersed in organic solvents, and dispersibility in polymer matrices is expected. Other polysaccharides have also been adsorbed on the surface of cellulose nanofibrils. Galactoglumanan has been used to functionalize CNFs in water, to impart hydrophobic behaviour (Lozhechnikova et al. 2014) or zwitterionic-xyloglucan block copolymers to produce super-absorbing films (L. Hatton et al. 2017). Polyelectrolytes were adsorbed on the surface of CNFs and used as linkers to introduce antimicrobial silver nanoparticles (Martins et al. 2012). The electrostatic assembly obtained Nanocellulose-silver nanocomposites in aqueous solutions. Polyacrylamide was used to modify the surface of cellulose fibres in a layer-by-layer assembly process to improve their hydrophobicity (Li et al. 2012a). Cationic polyacrylamide (CPAM) and sodium lignosulfate (LS) multilayers were formed on the surface of originally negatively charged cellulose fibres, after successive immersion in an aqueous solution of oppositely charged CPAM and LS. Electrostatic interactions were also harnessed for polyelectrolyte adsorption on nanocellulose by Larsson et al. (2013); Sankar et al. (2016); Brockman and Hubbe (2017) and Pötzinger et al. (2018).
Silylation The silylation with alkoxysilanes and their mechanisms of hydrolysis and condensation have been extensively studied for cellulose fibres (Brochier Salon et al. 2005). Indeed, aqueous silanols favour the adsorption on cellulose, and then the grafting occurs with dehydration during drying. Saini et al. grafted three different amino silanes in aqueous solutions on CNF films for antibacterial applications (Saini et al. 2017). Aminopropyl trimethoxy silane (APMS) was grafted onto a CNF suspension in water by Reverdy et al. (2018) and the hydrophobized CNFs were used in coatings formulation. Flame-retardant cellulose nanocrystals were obtained after silylation with methyltrimethoxysilane (Kim et al. 2018a). The reaction was performed in an aqueous acidic solution for 2 hours and followed by water elimination with freeze-drying. Similarly, aminopropyl trimethoxysilane was grafted onto CNCs (Khanjanzadeh et al. 2018). Good thermal stability was observed, and the hydrophobized CNCs can be used in composite applications. Triethoxy(3-glycidyloxypropyl)silane was grafted on the surface of cellulose nanofibrils in water under acidic conditions (Yeo et al. 2017). The modified CNFs were used to reinforce epoxy composites. To remove metal ions from water, 3-aminopropyl-triethoxysilane was grafted on CNFs in an ethanol-water solution after a 2 hour reaction time.
Etherification Etherification is another method to modify the surface of nanocellulose, and it has usually been performed in aqueous alkaline solution. Trimethyl ammonium groups were introduced on the surface of cellulose nanofibrils through nucleophilic addition of the cellulose hydroxyl groups to the epoxy group of the glycidyl trimethylammonium chloride (Najib and Christodoulatos 2019). The modified CNFs were able to adsorb arsenic from contaminated water. Etherification has also been used to graft epoxypropyl trimethylammonium chloride onto CNCs (Hasani et al. 2008).
Amidation Another way to modify cellulose in water is to graft on other moieties than hydroxyl groups. This multi-step approach is used onto the cellulose that has been oxidized into aldehydes then carboxylic acids. Oxidized nanocellulose was grafted with primary amine-containing compounds. In a mild procedure, the peptidic coupling can be performed in water in the presence of coupling agents, such as hydrochloride 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) or N,N’-dicyclohexylcarbodiimide (DCC). The amide coupling reaction involves EDAC as a catalyst and NHS as an agent to prevent the formation of stable N-acylurea that would reduce the reaction yield. Amidation with thermoresponsive Jeffamines copolymers was used to improve the steric stabilization of CNCs at high ionic strength and to generate thermoreversible aggregation (Azzam et al. 2010). Cysteine was grafted on TO-CNFs to develop microfiltration filters of thiol-CNFs embedded in polyacrylonitrile to adsorb metals (Yang et al. 2014). Water presents great advantages of non-toxicity and ease of use. However, the use of water as a solvent limits the range of chemical reactions and reagents available and washing steps in an organic solvent is sometimes required.
New solutions for nanocellulose functionalization
To expand the modification strategies with apolar molecules in a sustainable approach, by avoiding the use of organic and toxic solvents, new strategies have been investigated.
Solvent-free The first strategy to avoid the use of organic solvents is to develop solvent-free methods of cellulose modification. The molecule to be grafted is in direct contact with the nanocellulose and can even play the role of the solvent.
For example, Espino-Pérez et al. developed an esterification method called SolReact, in which the grafted molecule plays the role of the solvent and reactive agent (Espino-Pérez et al. 2014). A series of aromatic carboxylic acids (benzoic acid, phenylacetic acid, benzylacetic acid) was grafted on the surface of cellulose nanocrystals. The first step of water evaporation from the CNC suspension and carboxylic acid mixture is performed in a closed distillation process. The reaction was carried out during 20 hours at 130 °C, a temperature higher than the boiling point of water and the melting point of the carboxylic acids. Excess reactants were purified by distillation. A degree of substitution of 50 and 30 molecules of carboxylic acid per 100 AGU was reported for benzylacetic acid and phenylacetic acid, respectively. Benzoic acid grafting was ineffective due to the carboxyl double bond delocalization, limiting the reactivity of the carboxylic acid. In their approach, the esterification was considered as carried out in ‘one-pot’ as the solvent is the reactant. Additionally, the drying step, that has been previously used in other solvent-free techniques to create the covalent bond is here avoided. A variant of the SolReact protocol was proposed for the silylation of CNCs with trimethoxy(phenyl)silane (Espino-Pérez et al. 2016). This method was also used by Smyth et al. (2018) to functionalize CNCs with 4-pentenoic acid in an environmental-friendly esterification process, and the unreacted acid was removed by washing with ethanol. In a similar approach, CNCs were acetylated with acetic anhydride as the reagent and reaction medium in the presence of a citric acid catalyst (Ávila Ramírez et al. 2017). CNC powder was mixed with acetic anhydride and citric acid. Single-step esterification took place at 120°C during 3 hours and washed to remove unreacted reagents. FTIR and 13C NMR analysis confirmed the ester bond presence. The hydrophobized CNCs dispersed well in chloroform.
Solvent-free methods have also been developed for functionalization of nanocellulose by silylation. A mercapto silane was grafted on the surface of oxidized cellulose nanofibrils during a freeze-drying process (Geng et al. 2017). The procedure involves direct freeze-drying of the TO-CNF suspension in the presence of hydrolyzed 3-mercaptopropyl-trimethoxysilane. The functionalized cryogels were used for the selective removal of mercury ions from water, enhanced by the high amount of thiol groups. The removal efficiency of mercury was up to 93 % even after several adsorption-desorption cycles. More recently, Chantereau et al. deposited a (3-Aminopropyl)-trimethoxysilane and a (2-aminoethyl)-3-aminopropyl-trimethoxysilane on top of bacterial cellulose membrane (previously dried or wet) and successful silylation was achieved in a freeze-drying procedure (Chantereau et al. 2019). The modified bacterial cellulose exhibited antibacterial activity against S. aureus. The condensation of silanols with the hydroxyl groups of nanocellulose was performed at low temperature during sublimation of water.
Gas-phase and chemical vapour deposition . Gas-phase modification of cellulose avoids the use of solvents and limits the cleaning steps for recovery of the functionalized material. The reagents are brought in contact with the surface to be modified using vaporization. Gas-phase esterification was first performed by Yuan et al. (2005) on filter paper and tunicate CNC films. Dried samples were placed in a glass vessel in the presence of trifluoroacetic anhydride (TFAA) and acetic acid (AcOH) or TFAA and acetic anhydride (Ac2OH), at room temperature and during 15 to 120 minutes. After esterification, the structures exhibited a significant increase in hydrophobicity. Gas-phase esterification of CNFs films with vapour mixture of TFAA/AcOH or TFAA/Ac2OH was also used to increase the hydrophobicity and potentially improve the barrier properties of the film (Rodionova et al. 2013). The process is illustrated in Figure 26 (left).
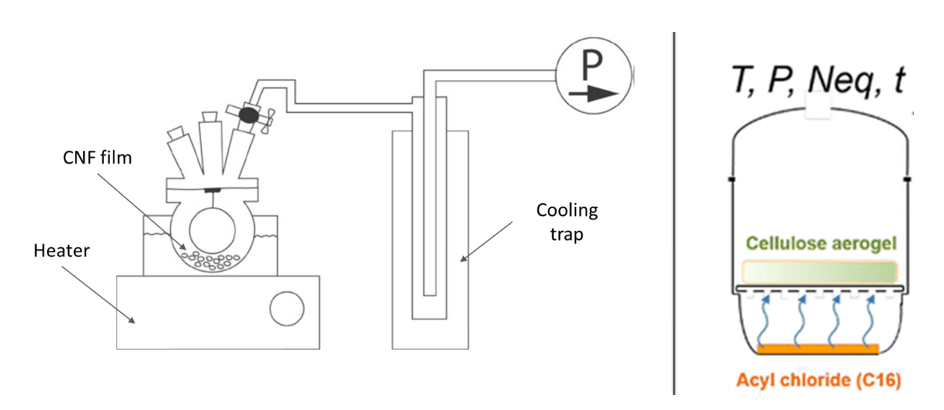
Figure 26: Gas-phase esterification process. From Rodionova et al. (2013) (left) and Fumagalli et al. (2013a) (right).
Esterification of model cellulose nanocrystals from tunicate and bacterial cellulose was performed in gas-phase with palmitoyl chloride by Berlioz et al. (2009). Nanocellulose were dried by freeze-drying or critical point drying and placed in a vessel, above the palmitoyl chloride reagent. The vessel was placed in a vacuum at controlled temperatures from 160 to 190°C. When the temperature was increased, the reagent evaporated and diffused in the porous nanocellulose material. Ester bonds were created between the cellulose hydroxyl groups and the acid chloride from the palmitoyl chloride vapours, and hydrogen chloride was released. A nitrogen flow eliminated the hydrogen chloride formed. Esterification was performed during given periods of time and followed by Soxhlet extraction for purification. Heterogeneous derivatization was confirmed by solid-state 13C NMR and X-ray diffraction. Very high temperatures (160 to 190 °C) and long reaction times (2 to 16 hours) were tested. The highest degree of substitution of 1.17 was achieved at 170°C and 13h reaction time for tunicate CNCs and was of 2.7 at 170°C for 6 h reaction time for bacterial cellulose. In a similar process illustrated in Figure I. 26 (right), CNF cryogels were modified by gas-phase esterification with palmitoyl chloride (Fumagalli et al. 2013a). The amount of reagent, the temperature, the pressure and the time were varied, and degrees of substitution from 0.14 to 2.6 were obtained. Low degree of substitution yields surface-functionalized CNFs exhibiting hydrophobicity while no alteration of the inner surface was observed. Similarly hydrophobized CNCs exhibit good dispersibility in hydrophobic non-polar solvents (Fumagalli et al. 2013b). Esterified CNCs with degrees of substitution up to 0.8 were not altered in their inner core and formed stable colloidal suspensions. The production of high specific surface area cryogels was essential for esterification with palmitoyl vapours. This gas-phase esterification process was extended to fatty acids with various aliphatic tail lengths, various reactive groups (acyl chloride and anhydride) and bi-functional fatty acids (Fumagalli et al. 2015).
Chemical vapour deposition (CVD) is a process in which the reagent in the vapour phase is deposited on the surface of the material to be treated. The process is performed at atmospheric pressure, low-pressure of ultrahigh vacuum, with or without plasma-enhancement and has been developed mainly for the textile industry (Malkov and Fisher 2010; Aminayi and Abidi 2015).
Trichloro(1H,1H,2H,2HtridecafluoroN-octyl) silane (FOTS) was deposited onto nanocellulose-coated paper in a chemical deposition process (Phanthong et al. 2016). The resulting paper exhibited both superhydrophobic and oleophobic properties with a contact angle of 156° and 144° for water and n-hexadecane.
The techniques of gas-phase functionalization have been used for porous materials, with complete wetting of the structure. However, the process requires high temperatures, where many compounds suffer thermal degradation and considerable amounts of energy and reagents are needed. It presents the disadvantage of requiring high vapour pressure chemicals but also to have highly porous and dried materials. This last point limits the use of this strategy with nanocellulose, especially if we want to redisperse the dried and functionalized nanocellulose materials.
Supercritical solvents . Another option for the replacement of organic solvents is the use of environmentally benign solvents such as supercritical solvents. The supercritical state of a fluid is reached when compressed above its critical pressure and heated at temperatures higher than its critical temperature. The critical values vary from a fluid to another, and some of them are reported in Table 3.
The use of supercritical fluids remains limited because of the necessity of suitable equipment and the high costs implemented. A major exception is observed for supercritical carbon dioxide (scCO2). Its non-toxicity and low critical parameters have increased its interest and applicability at industrial scale. Examples of the use of supercritical carbon dioxide include the food industry (caffeine extraction), pharmaceuticals (extraction of active principles, nanoscale particles production), dry cleaning, dyeing etc. For these reasons, more details of the properties and applications of scCO2 will be discussed below.
Table 3 Critical values of various fluids.
Fluid Tc (°C) Pc (MPa)
Ammonia 132.4 11.35
Carbon dioxide 31.1 7.38
Ethanol 241 6.3
Hydrogen -239.9 12.9
Nitrous oxide 36.6 7.24
Propane 96.8 4.25
Water 374.4 22.1
Supercritical carbon dioxide
Supercritical carbon dioxide has unique properties that combine those of gas and liquids (Lumia 2002). Small changes in the temperature and pressure result are large changes in density. The range of reachable densities is reported in Figure 27 (left). As illustrated in Figure 27 (right) scCO2 has gas-like low viscosities, resulting in rapid transfers inside the matrices, and can reach densities close to the densities of liquids. Finally, the diffusivity is in between one of the liquids and gases. This higher diffusivity than liquids is of particular interest for efficient mass transfer, compared to classical solvents.
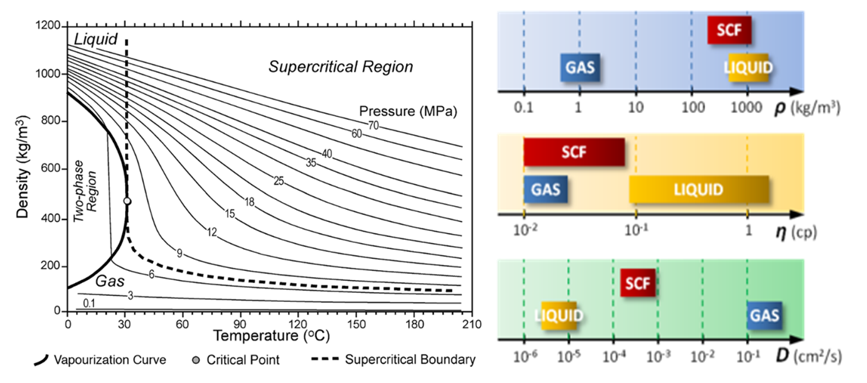
Figure 27: Supercritical carbon dioxide densities from (Bachu 2003) (left) and properties of density, viscosity and diffusivity (right).
Supercritical carbon dioxide is often discussed as a green alternative to organic solvents. It is a good solvent for low molecular weight, nonpolar molecules. Because of its non-toxicity, it is approved by the food and drug administration (FDA) and generally regarded as safe. It is considered as an environmental-benign solvent and is a by-product of some industrial processes. It is available in large amounts and high purity at a low cost. A great advantage of the use of scCO2 is the easy solvent recovery and product isolation and purification during depressurization. Another interesting property of scCO2 is its non-protic behaviour and its chemical inertness which could allow the use of reactive reagents.
Supercritical carbon dioxide has been used for the derivatization of cellulosic materials. However, very few papers report the functionalization of nanocellulose-based materials in supercritical conditions. For this reason, the literature review was extended to functionalization of cellulose-based materials and cellulose-derivatives materials. Two main types of functionalization strategies are distinguished: impregnation or coating of an active compound and covalent grafting.
Impregnation in scCO2 . Supercritical carbon dioxide has been used for impregnation of cellulosic materials with compounds of interest to confer various properties. Hydrophobization of cellulose has been performed by supercritical impregnation. Ammonium palmitate was impregnated on cotton fabrics to implement water repellant properties by Bilalov et al. (2017). The process was performed in supercritical carbon dioxide at pressures from 9 to 25 MPa and temperatures from 35 to 60°C. After a solubility study of ammonium palmitate, impregnation was performed on five cotton fabrics, and contact angles higher than 150 °C were reported. Russler et al. modified bacterial cellulose aerogels with alkyl ketene dimer (AKD) in supercritical CO2 at 40°C during 1 hour, at a pressure varying from 10 to 25 MPa. Samples above 30 % AKD grafted showed increase humidity uptake capacities. The process resulted in around 15 % of the impregnated AKD covalently grafted on the cellulose indicated in this case that the scCO2 is not inert (Russler et al. 2012). AKD was recently impregnated onto cellulose fibres to confer hydrophobicity (Adenekan and Hutton-Prager 2019). The development of hydrophobic paper was done in sub- and supercritical carbon dioxide. Impregnation during 15 minutes at pressures from 5 to 25 MPa was investigated. No covalent grafting was observed, and the AKD seem to be hydrogen-bonded to cellulose. Near-superhydrophobic properties were established 3 days after supercritical impregnation.
Dyeing of textiles in supercritical carbon dioxide overcomes the ecological problems of conventional dyeing and has been studied since the ’90s (Hendrix 2001). Reactive disperse dye with fluorotriazine reactive groups were synthesized, and their reactivity towards hydroxyl groups in scCO2 was investigated (Fernandez Cid et al. 2007). Cotton fabrics were pretreated in methanol before impregnation at 30 MPa, 120°C during 2 to 7 hours. Efficient dye fixation and deep shades were achieved, and no toxic by-products were produced, in contrast to the use of chlorotriazine dyes. Wool fabrics were dyed in supercritical carbon dioxide with azo, thiazole, anthraquinone/amide, and fluorescence dyes (Zheng et al. 2017). Adaptation of the temperature, pressure, time and CO2 flow led to high colour strength. Supercritical impregnation has also been used for wood dyeing by Jaxel et al. (2019). Prior AKD impregnation was shown to improve dyeing efficiency. Processes of supercritical dyeing are available at industrial scale, and the technology is used today by leading brands such as Ikea and Nike.
Active molecules of dexpanthenol and L-ascorbic acid were impregnated into bacterial cellulose aerogel by antisolvent precipitation (Haimer et al. 2010). Supercritical carbon dioxide was used to precipitate the molecule and dry the bacterial cellulose to yield bacterial cellulose aerogel loaded with bioactive compounds in one batch. The loading and release were controlled by the aerogel thickness and the amount of drug mixed to the bacterial cellulose prior drying.
Supercritical carbon dioxide has also been used as the impregnation medium to confer antimicrobial activity to cellulose samples.
Silicone with quaternary ammonium salts (QAS) was coated on cellulose substrates to confer antimicrobial properties (Chen et al. 2013). QAS silicone is highly hydrophobic, and their adsorption of on cotton swatches was performed in supercritical carbon dioxide at 25 MPa, 50°C during 3 hours. Activity against E. coli and S. aureus was observed in liquid growth conditions after 120, and 90 min contact with the coated cotton samples washed 50 times. In a similar approach, a surface-oriented fluorinated pyridinium silicone was impregnated in cotton yarns in scCO2 at 24 MPa, 50°C (Chen et al. 2018). The pyridinium silicon was highly CO2-philic and exhibited antibacterial properties. The stability of the coating and antibacterial activity of the coated cellulose was confirmed. Cotton fabrics were silver-coated in supercritical carbon dioxide to produce antifungal dressings (Gittard et al. 2010). Two types of silver complexes, (Ag(hepta) and Ag(cod)(hfac)), were dissolved in scCO2 at 210MPa, 40°C, in the presence of the cotton fabric and impregnation last for 10 to 15 hours. The process is illustrated in Figure 29a.
Supercritical carbon dioxide has been used for the extraction of essential oils. They have low water solubility and high solubility in scCO2. For this reason and their numerous interesting properties (antibacterial, anti-oxidant, anti-inflammatory), their impregnation in polymer matrices under supercritical conditions has been widely studied. Cotton gauzes were impregnated with thymol in scCO2 to provide antimicrobial activity (Milovanovic et al. 2013). Mild conditions of temperature (35°C to 50°C) and pressure (7.8 to 250 MP) were used in static conditions of impregnation. Impregnation yields from 11 to 19.6 % were achieved for impregnation times varying from 2 to 24 hours, and antimicrobial activity was confirmed against five microorganisms. Similarly, thymol had been incorporated into cellulose acetate and impregnation yields varied from 4.51 % to 72.26 % wt. (Milovanovic et al., 2015). High impregnation yields affected the morphology of cellulose acetate. Thymol was incorporated into poly(vinyl alcohol)-cellulose nanocrystals included into poly(lactic acid) (PLA) films under supercritical carbon dioxide conditions (Alvarado et al. 2018) at a thymol concentration of 198 mg.g-1 in poly(vinyl alcohol)- CNCs/PLA composites. Anti-biofilm formation properties were observed for cellulose acetate films impregnated with thymol in scCO2 (Zizovic et al. 2018). Other plant extracts have been impregnated under supercritical conditions, in collagen-cellulose dressings by da Silva et al. (2018) for anaesthetic wound dressings as illustrated in Figure I. 29d. Mango polyphenols, and more specifically, gallic acid as a model, was impregnated into cotton fabrics under supercritical conditions (Fernández-Ponce et al. 2018).
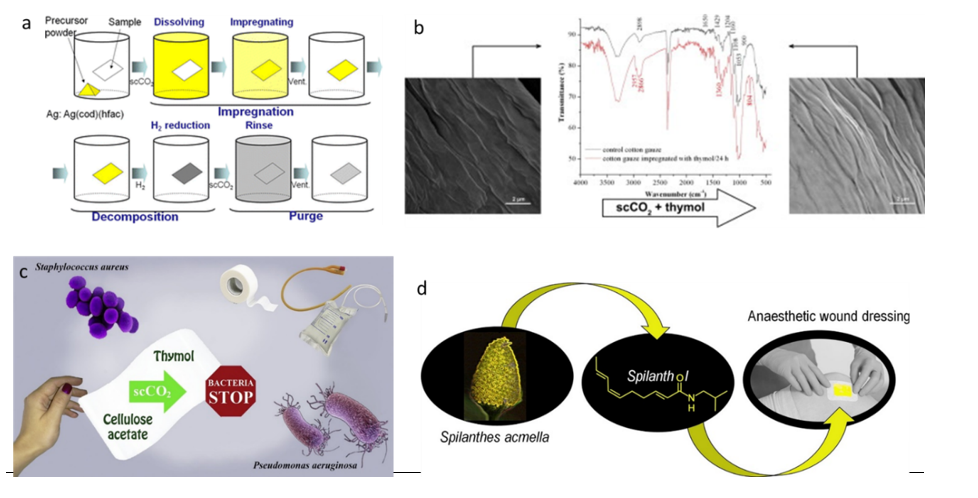
Figure 29: Supercritical impregnations to yield antimicrobial cellulose-based materials. Compilation of graphical abstracts from a: Gittard et al. (2010), b: Milovanovic et al. (2013), c: Zizovic et al. (2018) and d: da Silva et al. (2018).
Covalent grafting in scCO2
Many organic reactions are performed in supercritical solvents, and the wide range of chemistry has been reviewed (Oakes et al. 2001). Supercritical carbon dioxide solvent properties and solubility enhancement parameters have been reported by Peach and Eastoe (2014). However, only a few papers discuss the functionalization of cellulose materials and even fewer focus on nanocellulose modification in supercritical conditions.
Cellulose oxidation A method for the oxidation of cellulose has been developed in supercritical carbon dioxide. Regenerated cellulose was oxidized with nitrogen oxide in supercritical conditions (Camy et al. 2009). The degree of oxidation increased with decreasing pressures, and the NO2 concentration does not have a significant effect on the degree of oxidation. Carbon dioxide seemed to have an inhibiting effect on the reaction when its concentration increases. This may be due to a loss of water content that is needed to allow the oxidant to reach the amorphous zones. The NO2 mass fraction in the system had a positive impact on the reaction. The crystallinity of cellulose was maintained during the oxidation reaction in supercritical carbon dioxide. More cellulose degradation and loss of crystallinity were observed when the reaction was performed in N2. The pressure, temperature and initial water content were adjusted to control the degree of oxidation of cellulose. The process was patented and industrialized for the oxidation of cellulose and other polysaccharides (Vignon et al. 2006). However, NO2 is highly toxic. Moreover, the authors concluded that CO2 reacts with NO2, which makes this approach even less attractive.
Cellulose carbamates. Cellulose carbamates were synthesized in supercritical conditions by Yin and Shen (2007). Cotton pulp was placed inside a vessel in the presence of ethanol as a co-solvent and urea. ScCO2 conditions in the range 40-50 °C and 12-22 MPa were applied during 2 hours for impregnation of urea. After depressurization, a final step of heating at 132.7°C during 3 hours was carried out on the samples. The carbonyl peak was observed in FT-IR experiment and confirmed the reaction between urea and cellulose. XRD patterns of cellulose carbamates differed from native cellulose and could result from esterification changes in the crystalline structure of cellulose. Evolution of the rheological behaviour was also observed after esterification and depended on the nitrogen content. The same team prepared cellulose carbamate from lignocellulosic fibres (Zhang et al. 2013).
Acetylation Acetylation of cellulose fibres has been performed in supercritical CO2 as the reaction medium by Nishino et al. (2011). Ramie fibres were pretreated with glacial acetic acid and placed in the vessel in the presence of acetic acid in supercritical CO2 at 70°C, 9.8 MPa during 0.5 to 5 hours. A final step of vacuum drying at 60°C for 12 hours was needed before recovery of acetylated cellulose fibres. A degree of substitution of 2.2 was achieved, and acetylation reached the core fibres after 2-hour reaction time. When vapour phase treatment was performed, lower degrees of substitution and reaction rates were observed. No morphological changes were noticed from SEM images, and the crystallinity changes depended on the reaction time.
Copolymerization Graft copolymers of cellulose and poly(2,2,2-trifluoroethylmethacrylate) (PTFEMA) were synthesized in supercritical carbon dioxide (Liu et al. 2010). Ramie fibres were first modified in a traditional solvent to attach a chain transfer agent. The reversible addition-fragmentation chain transfer (RAFT) polymerization was carried out in supercritical carbon dioxide at 25 MPa, 70°C. The grafting ratios depended on the reaction time and resulted in highly hydrophobic surface materials.
Focus on the interest of supercritical carbon dioxide for silanization. Besides providing a good alternative to organic solvent and producing solvent-free materials, scCO2 can be used to produce homogeneous graftings with controlled configurations. Silanization, as a surface modification strategy, has been performed in scCO2 to yield homogeneous and uniformly functionalized surfaces (Sanli and Erkey 2015). Loste et al. (2004) compared silanization processes in conventional and supercritical solvents. The experimental set-ups and schematic representations of the reaction are illustrated in Figure 30.
The silanization reaction occurred in three steps. First, the alkoxy groups were hydrolyzed into silanols (Si-OH) in the presence of water or catalytic acidic or basic conditions. Then, the hydroxyl groups of the silanols formed hydrogen bonds with the hydroxyls of the substrate. Finally, covalent links were created during a curing step and concomitant loss of water molecules. Thus, the first step of silanization was the hydrolysis of the silane, in the presence of water.
In conventional solvent, silanes are also often hydrolyzed in bulk solution. Moreover, an acid or base is commonly added to catalyze the hydrolysis reaction. This is not necessary in the case of aminosilanes, for which the amino group catalyzes the reaction and neither for chlorosilanes that are highly reactive. In anhydrous organic solvents, the hydrolysis takes place only on the surface of the material. However, these anhydrous solvents are toxic (benzene, toluene) and/or carcinogenic (trichloroethylene, or choloroform). Other solvents like ethanol, methanol or acetone are rarely used in anhydrous conditions which leads to uncontrolled hydrolysis and silane polymerization. The aminopropyltrimethoxysilane or aminopropyltriethoxysilane is well known for forming polycondensation and obtaining a uniform monolayer with the amine group oriented away from the substrate can be a very sensitive and tricky task (White and Tripp 2000; Pasternack et al. 2008, p. 3).
In the supercritical media, silane hydrolysis is initiated by the presence of water as molecules adsorbed at the surface of the hydroxylated material. Because the solubility of water is low in scCO2, the water molecules remain on the surface. Hydrolysis is therefore initiated at the surface of the material only. Additionally, catalytic acidic conditions needed for silane hydrolysis are provided by the presence of water in scCO2. Indeed, the water in contact with CO2 has a pH close to 3 because of the formation and dissociation of carbonic acid.
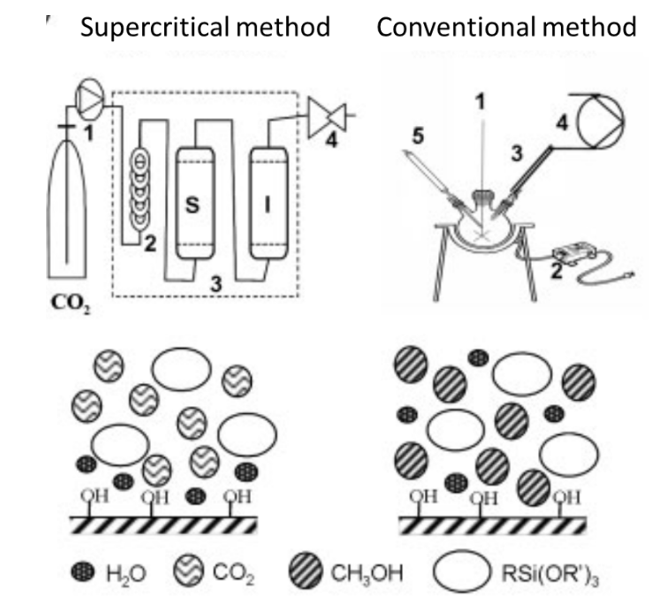
Figure 30: Experimental set-up of silanization in supercritical and conventional conditions and schematic illustration of the reaction (From (Loste et al. 2004).
These differences result in various grafting conformations. In conventional solvents, bulk hydrolysis of the silanes before deposition of the substrate promotes the presence of polycondensed structures. In supercritical carbon dioxide, polycondensation is prevented, and self-assembled and covalent monolayer structures are preferentially yielded.
As illustrated from this literature review, there are very few publications dealing with the functionalization on nanocellulose in supercritical carbon dioxide. This technology can extend the range of modification of nanocellulose, including foams, with apolar molecules. It also presents the advantage of being processed at mild conditions of temperature and pressure, being non-toxic and compatible with biomedical applications and yielding materials with no solvent residue.