Microorganisms or microbes are defined as living microscopic organisms. They are found in very different parts of the phylogenetic tree (Figure 14) including bacteria, fungi, protozoa and algae.
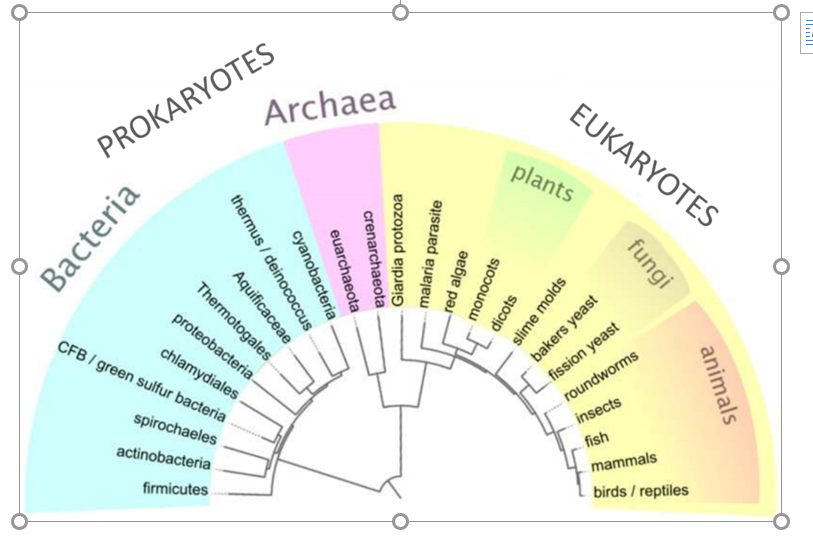
Figure 14: Phylogenetic tree of life of prokaryotes, covering both bacteria and archaea, and eukaryotes.
The microorganisms represent most of life on Earth and are essential to human life and the environment. They participate in the carbon and nitrogen cycles and are present in every ecosystem and play major roles such as decomposition and decay. They are present in the human microbiota where they are essential for digestion. However, some microorganisms are pathogens responsible for infectious diseases. The different morphologies and sizes of various microorganisms are presented in Figure 15. Viruses are sometimes considered as a form of life even though they do not have a cellular structure nor their own metabolism. Bacteria are prokaryotic unicellular organisms that are vital to many stages of the nutrient cycle on Earth. Yeasts are eukaryotic, unicellular organisms, belonging to the fungus kingdom. They are sometimes confused with moulds, which are multicellular fungi.
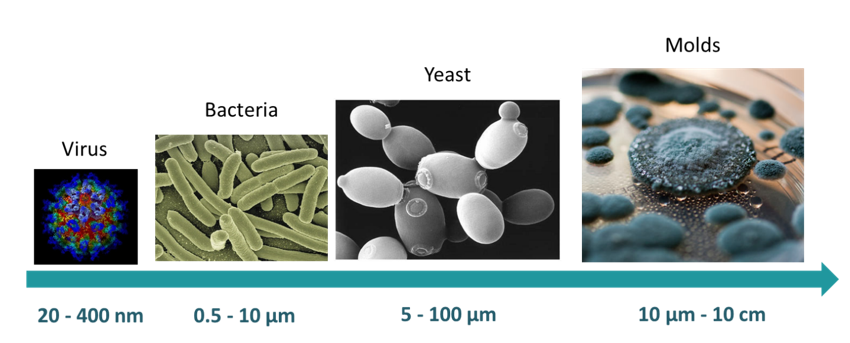
Figure 15 Average sizes and varied morphologies of microorganisms.
The composition and growth mechanisms of bacteria and yeast will be described in the following sections. Then, the pathogenicity of these microorganisms will be described followed by the antimicrobial agents.
Bacteria are unicellular, prokaryotic microorganisms, meaning they possess one unique chromosome without nuclear membrane. The prokaryotic chromosome is located in an irregularly shaped area of the cytoplasm, called the nucleoid. Some bacteria have the capacity to form spores, a dormant form of bacteria resistant to hostile environments. The different morphological properties can be studied by optical microscopy analysis. The dimensions of bacteria vary from 1 to 10 µm in length and present different shapes. Two main morphologies are observed: spherical called coccus and rod-shaped called bacillus. Cocci are present in pairs (diplococci), groups of four (tetrads), chains (streptococci), clusters (staphylococci) or cubes (sarcinae). Bacilli can assemble in pair (diplobacilli) and in chains (streptobacilli). The shape is linked to the membrane structure and has an influence on the nutrient uptake, motility of the bacteria, adherence or predation (Young 2007). Usually, staining of the bacteria is sometimes necessary for better contrast, when observing them with optical microscopy, especially to accentuate morphological specificities.
The cytoplasmic membrane limits the cytoplasm of the bacterium. It is very thin and made essentially of proteins (70 %) and lipids (30 %). The membrane is impermeable to various molecules (amino acids, sugars, proteins, nucleic acids) due to its lipophilic behaviour. The cellular respiration takes place in the cytoplasmic membrane. The membrane proteins allow active and passive transport of substances. The cytoplasm is a gel in which different elements can be found. RNA and ribosomes play a role in proteins synthesis. Reserve substances including glycogen, polyphosphates, iron, pigments are also found in the cytoplasm. The genetic material is composed of plasmids and chromosomes.
In 1884, Christian Gram developed the differential Gram staining method, which involves two staining steps, separated by ethanol washing (Gram 1884). Bacteria are separated into two major groups according to their response to the Gram-stain protocol: Gram-positive (G+) and Gram-negative (G-). Crystal violet is used to stain the cytoplasm of both types of bacteria. An iodine solution is added, and a large iodine-crystal violet complex is formed. The stain is washed with ethanol: Gram-positive bacteria remain violet while Gram-negative bacteria lose their colour. Finally, a counterstain is added and stains the decolourized G- bacteria in pink. This difference is due to the presence of large peptidoglycan in G+ bacteria that acts as an impermeable barrier to ethanol, preventing colour loss in the presence of ethanol. G- bacteria have a thin layer of peptidoglycan that is degraded by the decolourizer agent, and the cell is unable to retain staining. The peptidoglycan is composed of a glycan backbone, peptide chains and peptide cross-linkers and is responsible for the strength of cell walls. An outer membrane is found in G- bacteria only. It is composed of lipids, proteins and lipopolysaccharides specific to bacterial sub-species. The different membrane organization and staining are presented in Figure 16.
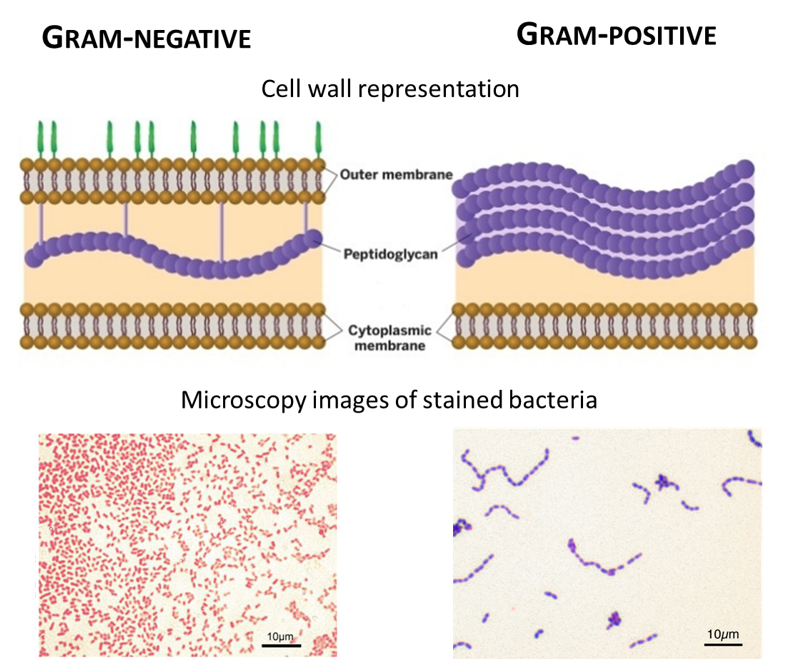
Figure 16: Cell membrane schematic representation and microscopy images of Gram-positive and Gram-negative bacteria. Images from microbiologyinfo.com.
Bacteria possess structures that extend from the cell surface into the environment. Pili are fibres made of proteins and adhesins and play a role for adhesion and attachment to surfaces. Conjugation pili are used for the transfer of genetic material. Flagella are long appendages enabling locomotion by rotation movement of the flagellar motor. Motility is dependent on the chemical nutrient gradient. The glycocalyx is an outer layer secreted outside of the cell wall. It is composed of polysaccharides and proteins and plays a role in adhesion and protection from the environment.
The growth of bacteria depends on the oxygen supply, and they are separated into three groups depending on their needs. The aerobic bacteria or obligate aerobes require oxygen. Obligate anaerobes, on the contrary, cannot survive in the presence of oxygen. Finally, many bacteria are neither aerobes nor anaerobes. Those facultative anaerobes can live with or without oxygen. Several other factors are influencing the growth of bacteria such as the temperature and pH. More information can be found in Alcamo’s fundamentals of microbiology book (Pommerville 2007).
When placed in a nutritive medium, either liquid or solid, the growth of a bacterial population follows four distinctive phases illustrated in Figure 17: a lag phase, a logarithmic phase, a stationary phase and a death phase (Madigan et al. 2007). In the very beginning, bacteria are adapting to their environment, and there is no increase in bacterial cells. When the active growth begins, an exponential increase in the number of cells is observed. Cell division is species-dependent and influenced by environmental conditions. After several hours of culture, a stationary phase is reached. The reproductive and death rate are similar. Eventually, the waste products accumulate, and when the amount of nutrient is limited, the population enters in a decline phase: the death rate is faster than the cell division and the number of living cells decreases.
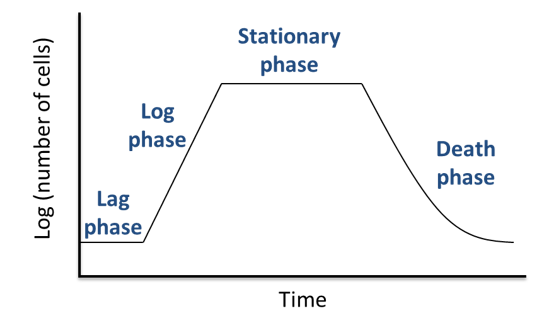
Figure 17: The different phases of bacterial growth.
Yeast are members of the fungus kingdom. Fungi are a diverse group of eukaryotic microorganisms. A eukaryote is defined by the presence of a nucleus enclosed within membranes as well as enclosed mitochondria and Golgi apparatus. More than 75000 fungi species are known, and the estimation of the actual number is a tricky task (Blackwell 2011). A recent paper estimated the number of species to 2.2 to 3.8 million (Hawksworth and Lücking 2017). There are three main groups of fungi: filamentous moulds, yeasts (usually unicellular) and mushrooms. Only moulds and yeast concerns microbiology. Yeasts are unicellular organisms and form colonies similar to bacteria colonies when growing on nutritive agar. For some species, dimorphism is observed: yeast form filamentous moulds at ambient temperature and unicellular organisms at body temperature. Being eukaryotic organisms, yeasts are defined by the presence of a nucleus enclosed within membranes as well as enclosed mitochondria and Golgi apparatus and the typical cell organization is presented in Figure 18. The cell wall is composed of chitin and provides rigidity. The mechanisms of fungal reproduction are described in Alcamo’s fundamental of microbiology book (Pommerville 2007).
Being eukaryotic organisms, yeasts are defined by the presence of a nucleus enclosed within membranes as well as enclosed mitochondria and Golgi apparatus and the typical cell organization is presented in Figure I. 18.
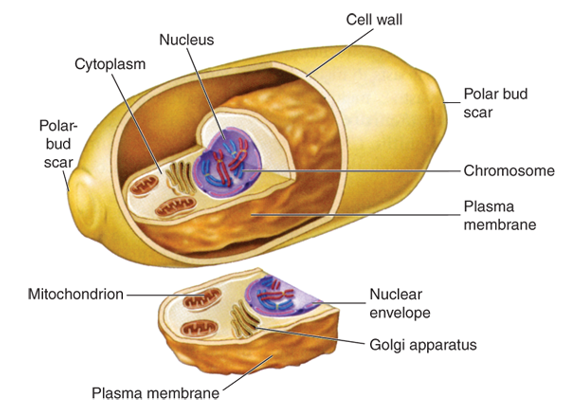
Figure 18: Representation of a fungi cell (Ryan et al. 2009).
Yeasts adsorb nutrient through the cell wall in a similar way bacterial cells do. Most fungi are aerobes except for some yeasts that are facultative anaerobes. Fungi generally have life cycles involving two phases: a growth (vegetative) phase and a reproductive phase. Their growth depends strongly on temperature and pH conditions. Reproduction of fungi involves spore formation. Some of them are harmful (Candida species, dimorphic fungal pathogens (Van Dyke et al. 2019)) while others have been used in the food and health industry (Saccharomyces boulardii helps to restore the natural gut flora, Saccharomyces cerevisiae for the production of beer and bread, comestible mushrooms such as Morchella species).
Pathogenicity of microorganisms
Microorganisms are classified into four different risk groups depending on their potential infection risk. This classification has been introduced in the labour legislation. The French National Centre for Scientific Research (CNRS) published guidelines for microorganisms handling according to their classification (CNRS 2017). Appropriate handling must be respected, and biological laboratories are classified into four corresponding safety levels with an increasing amount of precautions. To establish pathogenicity, factors such as host susceptibility and resistance, virulence factors or intracellular growth are taken into account (Peterson 1996).
In risk group 1 are found microorganisms that are unlikely to cause illness in human (or plants and animals), and that are already present in the environment. Bacillus subtilis is an example of group 1 bacteria. Handling can be performed on standard open benches; also, it is commonly performed under laminar flow hoods.
Risks group 2 microorganisms are susceptible to cause disease in humans (or plants and animals). However, the propagation in the environment of these microorganisms is unlikely and efficient treatments exist. These microorganisms must be handled by trained personnel only, in a biosafety cabinet and the laboratory must provide a suitable mean of decontamination (autoclave) and have lockable doors. Strains of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, Yersinia pestis and Mycobacterium tuberculosis are examples of risk group 2 bacteria and fungi.
In risk group 3, microorganisms cause serious diseases and present a risk for laboratory workers. There is a possibility of spreading in the environment, but preventive measures or treatment are available. Shigella dysenteria, Bacillus anthracis, SARS virus are group 3 pathogens. Registration with appropriate agencies is necessary to work with these agents.
Risk group 4, microorganisms are life-threatening and considered as a danger for human. There is a high risk of propagation and no efficient treatment. The highest level of biosafety precaution must be applied. The biosafety cabinet must not have sharp edges and be designed to facilitate decontamination and prevent glove damage. Research on Ebola virus, Lassa virus or poorly characterized dangerous pathogens is performed in class 4 laboratories. Until very recently no bacterial or fungal agents were found in risk group 4, but it now includes resistant Mycobacterium tuberculosis.
Microorganisms vary from one to another in term of morphology, growth environment or cellular organization. Only a few of them can cause human infection or illness. However, due to the seriousness of the disease they lead to, pathogenic species attract microbiologists focus on finding solutions to avoid spreading and treat infections.
Antimicrobial agents . Different terminologies are used to describe various antimicrobial activities. Sterilization is the term used to describe the process in which every microorganism is eliminated from the surface or an object. Bacteria, viruses, fungi and spores are killed. Chemical, physical or mechanical agents can be used for sterilization. Decontamination aims at eliminated most pathogens but not necessarily all the microorganism’s population. Disinfection reduces the amount of contamination from an inert material and from living tissues the term antisepsis is used. It is usually performed via chemical agents: disinfectants or antiseptics. Agents that kill the microorganism are microbicidal (bactericidal, fungicidal). When they reduce the number of microorganisms or inhibit their growth, they are bacteriostatic or fungistatic.
To assess the amount of an antimicrobial drug necessary to inhibit the growth of the microorganism, minimum inhibitory concentration (MIC) experiments are carried out. The MIC value is valid for a specific strain of microorganism. This value is given for in vitro activity. In vivo, the critical concentration will depend on the posology and route of administration. The method of determination involves a broth dilution assay. The Mueller-Hinton nutrient broth is a commonly used media as it does not present inhibitors towards most antibiotics. The antimicrobial drug is diluted into multiple culture wells to obtain a concentration gradient. The MIC is the concentration of antimicrobial drug for which a clear well is observed, indicating the absence of microorganisms. In Figure I. 19, the MIC of thymol was determined for a strain of E. coli used for quality control of antibiotic sensitivity by the European Committee on antibiotic susceptibility testing (EUCAST). A concentration of 250 µg.mL-1 of thymol was necessary to inhibit bacterial growth. Additionally, an increase in lag time (delay in growth) from 1 hour to 6 hours is observed for a concentration of 125 µg.mL-1 compared to the growth of bacteria without any drug in the culture media.
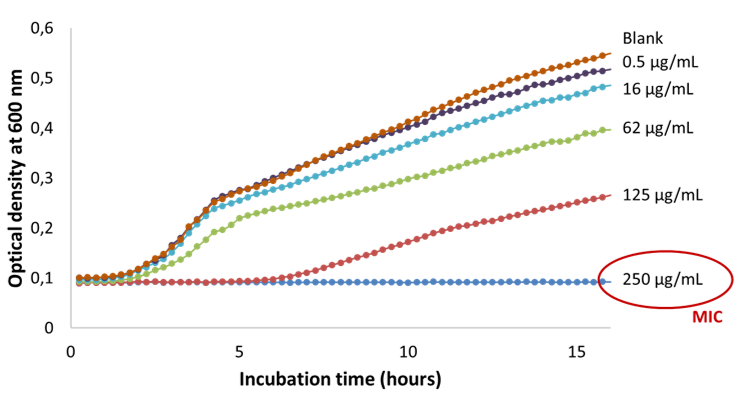
Figure:19 Determination of the minimum inhibitory concentration of thymol against E. coli).
To prevent food and water contamination, treat infectious diseases, destroy pathogens and prevent spreading, the use of antimicrobial agents is essential. Antimicrobial performance is based on physical, mechanical or chemical action, described in this section.
Physical agents.
Heat is the most commonly used method to control microorganism development. Microorganisms have an optimum temperature and are very sensitive to high temperatures. Their sensitivity varies from a species to another and is expressed as a function of time and temperature. The temperature has been used as an antimicrobial agent in processes of cooking, pasteurization, incineration, autoclave … Dry or wet heat can be applied depending on the substrate to be treated. Radiation is the second type of physical agent. Microwave and infra-red radiations have antimicrobial activity due to the thermic effect. Ionization (X-ray and gamma rays) leads to oxidative stress and irreversible damages to protein, deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and lipids. Ultraviolet light produces enough energy to modify RNA and DNA. Dehydration and/or osmotic pressure are used in food perseveration (salting) to prevent contamination. Low temperatures (refrigeration and freezing) are also used to retard contamination by reducing the growth rate of microorganisms (Pommerville JC, 2007).
Recently, supercritical carbon dioxide has been used for its bactericidal effect (Perrut, 2012). Different parameters seem to affect the microorganisms, and the mechanisms include cell wall rupture, enzyme inactivation, intracellular electrolyte balance perturbation. A rapid depressurisation or several pressurisation/depressurisation cycles improve the sterilisation under supercritical conditions. The presence of water in scCO2 leads to a decrease in pH, has a strong antibacterial effect (Nakamura et al. 1997). Mild conditions of temperature and pressure, from 30 to 80°C and 55 bars, are sufficient for bactericidal activity (Perrut 2012). For spore inactivation, more extreme conditions of pressure and temperature or the addition of hydrogen peroxide or trifluoroacetic acid are necessary. Thanks to the low toxicity of carbon dioxide, easy removal as well as its inert behaviour, supercritical sterilisation have been recently used for fragile and sensitive biomaterials and medical devices (Herdegen et al. 2014; Bernhardt et al. 2015; Scognamiglio et al. 2017; Soares et al. 2019).
Mechanical agents
Filtration is used to eliminate microbes from liquid media. Filters with appropriate pore sizes (0.2 or 0.45 µm) are used to trap the microorganisms. Air filtration with high-efficiency particulate air (HEPA) filter is performed in the biosafety cabinet. Soaps and detergents have both a chemical and mechanical action. They remove the microorganisms by entrapping them inside the micelles where they are eliminated with rinsing water.
Chemical agents.
They can be found in gaseous or liquid form and are listed in various categories.
Oxidising agents include hydrogen peroxide, iodine, chlorine and its derivatives (ex: sodium hypochlorite). When the production of oxygen reactive species is higher than the antioxidant power of the cell, damage to DNA, proteins and lipids are caused.
Metals ions, such as silver, gold and copper, present antimicrobial efficiency with high toxicity at low concentration (Turner 2017). However, the spread of resistance is observed and the widespread use of metal ions as antimicrobial agents present environmental toxicity issues. Quaternary ammonium compounds, such as benzalkonium chloride, have antimicrobial activity and are used as disinfectants. Their action targets the cell membrane stability, and they exhibit activity against bacteria, fungi and some viruses.
Antibiotics.
Antimicrobial agents are being used in vivo to treat infections. Antibiotics, discovered at the beginning of the 20th century, were derived from natural compounds and are now synthesised. They have a specific action against bacteria by altering an essential step of their development. Five main types of action are reported: inhibition of the cell wall synthesis, inhibition of cell wall function, protein synthesis inhibition, inhibition of nucleic acid synthesis, inhibition of other processes (Kapoor et al. 2017). Antibiotics have a narrow spectrum of activity against certain types of bacteria or a large activity; they are bactericidal or bacteriostatic and are active at low concentrations. However, they present toxicity and side-effects for the patient and their use must be controlled and adapted to the pathogen. Bacteria have developed natural resistance to antibiotics (Blair et al. 2015; Kapoor et al. 2017). Increase of bacterial resistance is partly due to the excessive and inadequate prescription of antibiotics. For these reasons, natural resources constitute a large supply of antimicrobial molecules to circumvent antibiotic resistance. There is historical evidence of the use of herbs, honey and other natural treatment to cure infections and prevent contamination. Natural molecules have proven to have antimicrobial activity.
Antimicrobial peptides
They are biosynthesised by unicellular and pluricellular organisms (animal, plants, bacteria, fungi) as a defence against pathogens invasions. They target the pathogen microorganism or boost the immune cells of the host.
Essential oils. Plants synthesize thousands of small molecules of the various constitution, most of which have antimicrobial properties (Gibbons 2008). Generally, their efficiency is lower than antibiotics. The minimum inhibitory concentration of an antibiotic is between 0.01 µg.mL-1 and 10 µg.mL-1. Phytochemicals have higher MICs between 100 µg.mL-1 and 1000 µg.mL-1. Essential oils are secondary metabolites. They do not play a role in plant growth mechanisms but offer antioxidant action, anti-inflammatory effect and natural protection against microbial invasion. Many studies report antibacterial action against Gram-positive and Gram-negative bacteria as well as the antifungal activity of essential oils molecules (Singh et al. 2012; Marchese et al. 2016; Bassanetti et al. 2017; Dias et al. 2017). Moreover, their activity is extended to antibiotic-resistant strains (Magi et al. 2015; Yadav et al. 2015). Terpenoids and phenylpropanoids are the components of the essential oil that confer antimicrobial activity. Kalemba and Kunicka listed essential oils component depending on their activity intensity (Kalemba and Kunicka 2003). Some active essential oils molecules are presented in Figure 20. Their activity is considered to be due to a hydrophilic/lipophilic balance that destabilizes the cell walls of microorganism. The mode of action is specific to a molecule/microorganism pair and is not always fully understood (Marchese et al. 2017; Vasconcelos et al. 2018; Cai et al. 2019). Phenolic and carbonyls compounds (thymol, eugenol, carvacrol, cinnamaldehyde) generally have high antimicrobial activity.
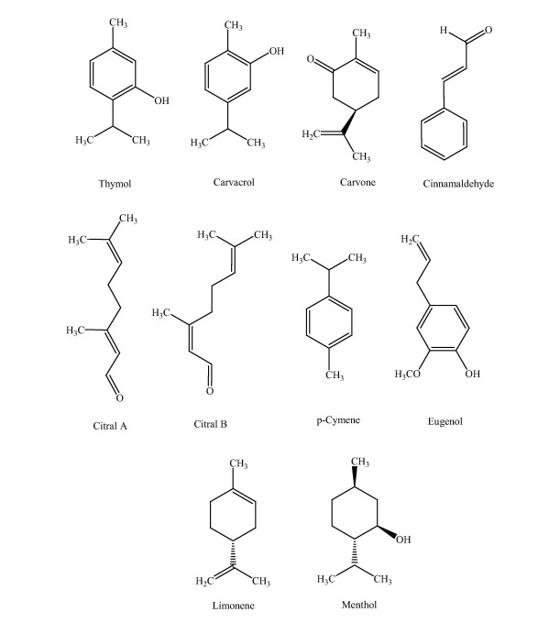
Figure 20: Essential oils compound with antibacterial activity (Marchese et al. 2016).
Antimicrobial agents are used for various type of infections (fungal and bacterial), lung infections (respiratory tract), blood, gastrointestinal, nervous system, generalized infection, dental, and topical infection. Skin infection could be due to dermal diseases or wound infections. The latter are reviewed in the following section.
