CELLULOSE NANOCRYSTALS (CNCs)
Cellulose nanocrystals are rod-shaped particles of high crystallinity. They are isolated from the acid hydrolysis of the disorderd regions of the parent cellulose microfibrils, under controlled conditions of acid concentration, cellulose to acid ratio, temperature and time. Nickerson and Habrle first isolated them in 1947 (Nickerson and Habrle 1947) by cellulose degradation in boiling acidic conditions (2.45 N hydrochloric acid and 0.6 M ferric chloride), and a few years later Rånby obtained stable CNC suspensions thanks to the introduction of negative surface charges (Rånby 1951).
CNCs have dimensions of 100 nm to a few µm in length, and 4 to 70 nm in cross-section (Klemm et al. 2011). The dimensions of CNCs strongly depend on the cellulose source and, to a lesser extent, on the hydrolysis conditions (Elazzouzi-Hafraoui et al. 2008; Chauve et al. 2014). Transmission electron microscopy (TEM) images of cellulose nanocrystals isolated from plants, bacteria, algae and marine organisms are shown in Figure 3 (left). In Figure 3 (right) a table from Habibi et al. (2010) reports the lengths and widths of CNCs of different sources and determined by different techniques including TEM, atomic force microscopy (AFM), field emission gun scanning electron microscopy (SEM-FEG), small-angle neutron scattering (SANS) and depolarized dynamic light scattering (DDLS). As a consequence of the difference in dimensions, the aspect ratio of these nanoparticles, defined as the length-to-width ratio, which plays a major role in phenomena like the self-organization of CNCs into chiral nematic crystal-liquid phases or the percolation threshold that is a key parameter governing mechanical properties in nanocomposites, extends over a wide range, namely from 10 to 100.
When extracted from cotton, CNCs are short, highly crystalline rod-like particles. They consist of the assembly of laterally aggregated elementary crystallites (3-4 on average), and their average dimensions are 150 × 22 × 6 nm3. Tunicates are marine invertebrate organisms that produce cellulose in their external mantle. CNCs extracted from tunicate are needle-like particles with a much higher aspect ratio and near-perfect crystallinity. Acid hydrolysis of tunicates generally yields CNCs made up of a single crystallite. Localized defects are often observed and result from the sonication treatment (Figure 3,f). The size of CNCs extracted from tunicate is between 1 and 3 µm in length and 10-30 nm in width.
Sulfuric acid is commonly used as the hydrolyzing agent. The basic idea behind the extraction process is that the starting cellulose microfibrils are made up of highly crystalline regions connected through loosely packed regions that are much more susceptible to be hydrolyzed during a chemical acid attack. Therefore, after diffusion of the acid within the substrate, the glycosidic linkages in the disordered regions are preferentially broken, releasing rod-like cellulose crystallites in the medium. The use of sulfuric acid imparts a negative surface charge in the form of sulfate half-esters, which ensures colloidal stability in aqueous media thanks to repulsive electrostatic interactions (Revol et al. 1992). The surface charge content is typically in the range 100-350 mmol.kg-1, which corresponds to a charge density in the range 0.2-0.6 e.nm2. (Foster et al. 2018).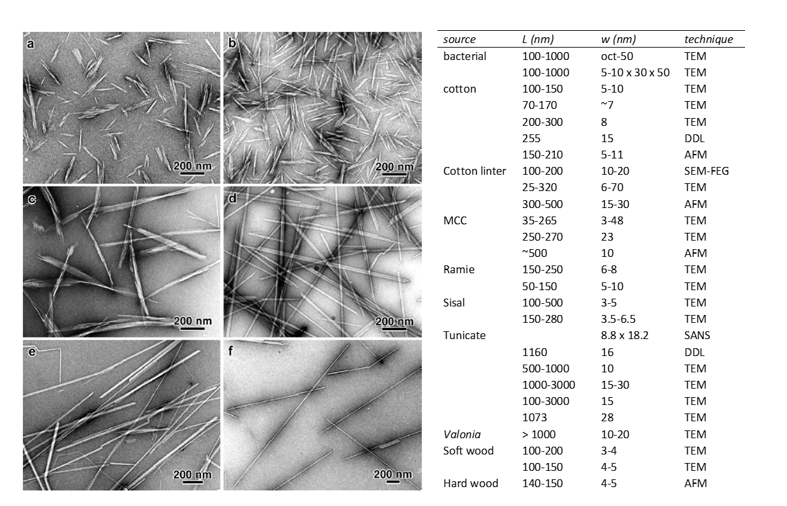
Figure 3: Transmission electron microscopy image of CNCs from cotton (a), ramie (b), Gluconobacter (c), Glaucocystis (algae) (d), Valonia (algae) (e), Halocynthia (tunicate) (f) from Martin (2015) thesis manuscript and dimensions of CNCs of different sources measured with a variety of techniques. Table from (Habibi et al. 2010)
CNCs are derived from an abundant and renewable source, cellulose fibres, and possess exceptional properties such as a vast surface area (250 m2.g-1), a low density (1.6 kg.m-3), and mechanical properties comparable to the one of Kevlar. Furthermore, these non-toxic and biocompatible nanoparticles are now produced at an industrial scale, thus paving the way for their commercialization and their availability in large quantities. Their characteristics have been widely reviewed (Habibi et al. 2010; Peng et al. 2011; George and Sabapathi, 2015; Trache et al. 2017; Grishkewich et al. 2017). Their industrial production and applications have also been the subject of numerous reviews (Habibi et al. 2010; Peng et al. 2011; George and Sabapathi 2015; Trache et al. 2017; Grishkewich et al. 2017)
CELLULOSE NANO FIBRILS (CNFs)
Different terminologies are used to describe cellulose nanofibrils (CNFs), such as nano-fibrillated cellulose (NFC), micro-fibrillated cellulose (MFC) or cellulose microfibrils or microfibers (CMF). The term cellulose nanofibrils (CNFs) was preferred by different organizations and committees (Technical association of the pulp and paper industry -TAPPI-, international organization for standardization -ISO- and the Canadian standards association -CSA) in an objective of standardization.
Cellulose nanofibrils form an opaque grey-white gel in water and are viscous suspensions even at low concentration. Unlike the rigid and highly crystalline CNCs, CNFs contain both amorphous and crystalline regions generating flexibility and possible entanglements. They have dimensions up to a few micrometres in length and 5 to 60 nm in diameter (Klemm et al. 2011). A photograph of a CNF suspension, as well as transmission electron microscopy and atomic force microscopy images, illustrate these properties in Figure 4.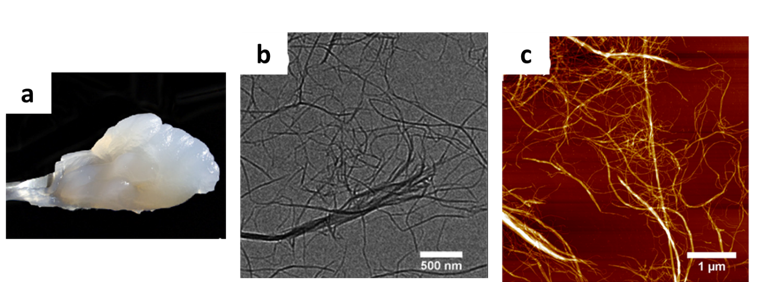
Figure 4: Photograph of a CNFs suspension (a) from (Lavoine et al. 2012a), TEM (b) and AFM (c) images of cellulose nanofibrils suspensions from (Sacui et al. 2014)
CNFs were originally produced by high-pressure homogenization to delaminate the fibres. This process was introduced by Turbak et al. (1983). Other mechanical processes have been used such as grinding, ball milling, refining, blending, extrusion, etc., and were described by Nechyporchuk et al. in their review (Nechyporchuk et al. 2016). To reduce energy consumption during the production of CNFs, pre-treatments methods have been developed. Chemical pre-treatments such as TEMPO oxidation (Saito and Isogai 2004), enzymatic attack (Lavoine et al. 2012b), cationization (Olszewska et al. 2011) or periodate oxidation (Kim et al. 2000, Kasai et al. 2014, Plappert et al. 2017) are also being used and lead to the production of CNFs with different surface chemistry and properties.
Enzymatically pre-treated CNFs
The enzymatic hydrolysis of the cellulose fibres favours the defibrillation, hence decreases the energy consumption during refining. The hydrolysis of cellulose is performed through cellulose-degrading enzymes called cellulase by three distinct processes. The hydrolysis of disaccharides and tetrasaccharides into glucose is performed by cellobiases, the hydrolysis of the amorphous regions of the cellulose by endoglucanases, and the enzymatic action at the end of the cellulose chains to release disaccharides and tetrasaccharides by exoglucanases. In 2007, Pääkkö et al. and Henriksson et al., reported new methods to produce CNFs involving a combination of enzymatic and mechanical pretreatments followed by high-pressure homogenization (Pääkkö et al. 2007; Henriksson et al. 2007). These enzymes, from Novozymes, composed of endoglucanases and exoglucananses, have been used in other studies (Turon et al. 2008; Siqueira et al. 2010b) and Nechyporchuk et al. investigated the use of different enzymes at different concentrations on the resulting CNF morphology (Nechyporchuk et al. 2015).
TEMPO-oxidized CNFs
The selective oxidation of primary hydroxyl groups of sugars using sodium hypochlorite in the presence of catalytic amounts of 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) radical in NaClO and NaBr was first studied by Davis and Flitsch (1993) and was optimized for nanocellulose by several teams for both cellulose nanofibrils and cellulose nanocrystals (Montanari et al. 2005; Habibi et al. 2006; Isogai et al. 2011; Rattaz et al. 2011).
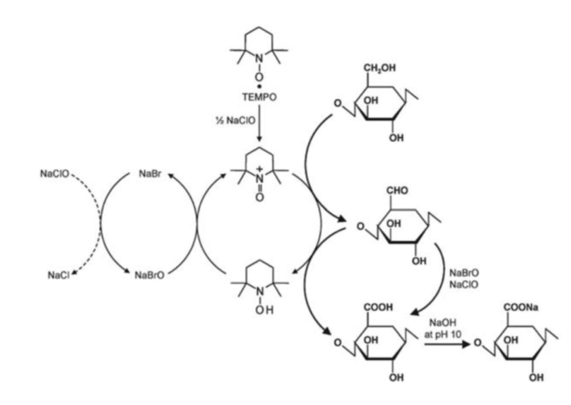
Figure 5: Selective oxidation of C6 primary hydroxyl of cellulose to carboxylate groups by TEMPO/NaClO/NaClO2 oxidation in the water at pH 10-11. (Isogai et al. 2011)
Saito et al. disintegrated various never-dried celluloses after TEMPO-oxidation and homogenization (Saito et al. 2006). The most energy-efficient procedures were deduced from the investigation of different reaction conditions (Isogai et al. 2011). Using such a process, negatively charged carboxylate groups are introduced at the surface of the cellulose chains. The resulting electrostatic repulsions promoted between the chains favour the defibrillation process during the mechanical treatment. The carboxylate content depends on the quantity of oxidizing agent used and Isogai et al. reported maximum values of 1.7 mol.kg-1 (Isogai et al. 2011). An alternative process at pH 6.8 instead of pH 10 was developed to limit the inevitable significant depolymerization that occurs at pH 10 (Saito et al. 2009).
CNFs share common properties with CNCs such as lightweight character, abundant and renewable origin, high specific surface area or broad chemical modification ability but also exhibit specific characteristics such as high viscosity due to the very high aspect ratio and entanglements and ability to form homogeneous flexible films (while short aspect ratio CNCs rather form brittle films). They also exhibit high modulus and tensile strength. They show interesting optical properties (liquid crystalline behaviour of CNCs and the ability to form transparent films with both CNCs and CNFs) and present potential compatibility with other materials and living cells. CNCs and CNFs have been investigated for toxicity assessments. Lin and Dufresne reviewed toxicology evolution of CNCs, CNFs and bacterial cellulose (Lin and Dufresne 2014). In vitro and in vivo experiments have been performed, and no or low cytotoxicity was observed, and no serious environmental concerns were raised. In the two worst-case scenarios, inflammatory cytokines and pulmonary inflammation were reported. The options of nanocellulose modification and processing are extremely versatile and open up a wide range of functions and structures (Abitbol et al. 2016).
APPLICATIONS OF NANO-CELLULOSES
The various and interesting properties of both CNCs and CNFs open up groundbreaking application areas (Abitbol et al. 2016). Over the last decade, the production of both types of nanocellulose at the industrial scale has increased their interest in both research and industry.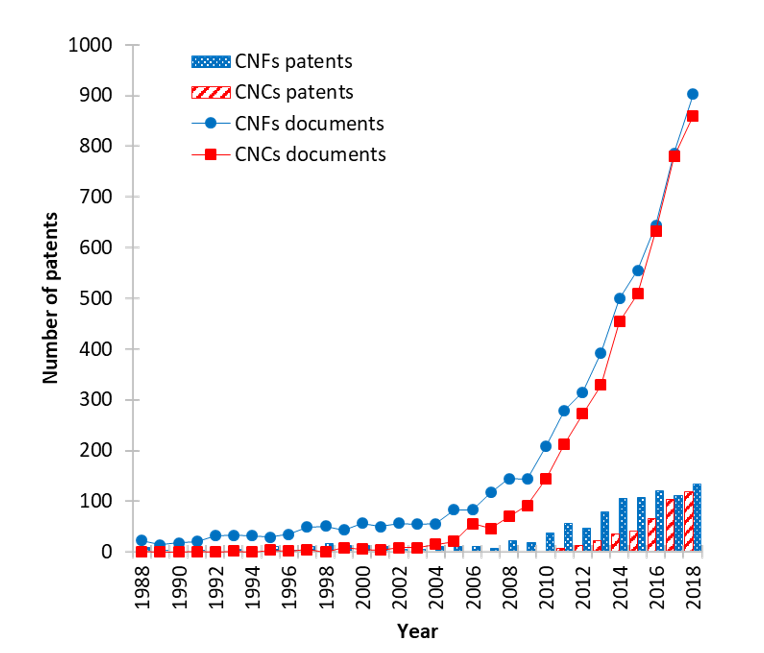
Figure 5: Number of documents (articles, conference papers, review and book chapter) from Scopus in July 2019 and patents (from SciFinder, June 2019) released each year dealing with CNFs (blue) and CNCs (red), descriptors for CNFs: cellulose nanofibrils/cellulose microfibrils / nanofibrillated cellulose / micro-fibrillated cellulose and for CNCs: cellulose nanocrystals/cellulose whisker /cellulose nanowhiskers / nanocrystalline cellulose
The number of patents with nanocellulose expanded when their production was no longer restricted to pilot scale. Studies from Reid et al. (2017) and Desmaisons et al. (2017) compared the properties of different CNCs and CNFs obtained from industrial and lab-scale productions. These works emphasize the existence of different grades of CNCs and CNFs and the importance of a thorough investigation of the properties of the commercial product beforehand.