Porous nanocellulose materials have been prepared with various properties to meet the requirements for a wide range of applications, including the biomedical field.
In the energy field, cryogels are used in solar cells as efficient and inert electrolyte deposition systems (Miettunen et al. 2014), and aerogels are used in the design of energy storage devices (Zu et al. 2016) exhibiting good charge-discharge rates. The low thermal conductivity of cellulose also makes it a good candidate for the design of thermal insulating cryogels (Jiménez-Saelices et al. 2017a). The ultralight properties of nanocellulose foams combined with good combustion resistance were used to design cryogels with good thermal insulation and fire-retardant properties (Nguyen et al. 2014; Han et al. 2015). Modified cryogels have been developed for oil/water separation and selective absorption capacities that can be used for depollution (Zhang et al. 2014). Highly porous hydrophobized CNF cryogels have demonstrated good efficiency in the absorption of non-polar liquid from polar media, with a capacity of up to 45 times their own weight (Cervin et al. 2012). Such cryogels have also proven to be reusable, with high absorption capacities up to 30 absorption/desorption cycles (Zhou et al. 2016).
Aerogel and cryogel materials are of particular interest for biomedical applications, and many of them are reported in a review by Stergar and Maver (2016). Porous aerogels are widely used for tissue engineering, drug delivery or wound dressings applications. A review from García-González et al. (2011) presents case studies of aerogels prepared from bio-based polysaccharides such as chitin, alginate and cellulose. Because of the low toxicity and biocompatibility of the nanocellulose materials as well as their water holding capacity, nano cellulose are excellent candidates for biomedical applications and many examples of nanocellulose implants, wound care, and drug delivery systems are illustrated in Jorfi and Foster’s (2015) recent review. The amount of publication per year on these topics up to 2013 has been established by Jorfi and Foster (2015). An extension until 2018 of these results was carried out and is presented in Figure I. 13. In the last five years, the amount of documents on drug delivery and wound healing remains almost constant; however, a major increase on the topics of antibacterial and antimicrobial activity can be observed.
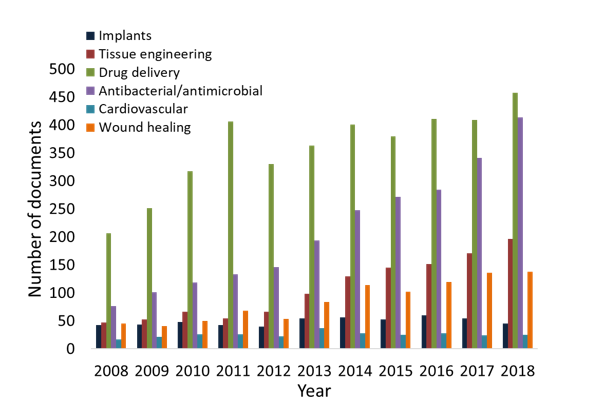
Figure 13: Number of documents in the period 2008-2018, from Scopus, July 2019 with descriptors: cellulose AND implants, tissue engineering, drug delivery, antibacterial/antimicrobial, cardiovascular and wound healing. Data originally from (Jorfi and Foster 2015).
To use nanocellulose and nanocellulose-based structures for biomedical applications, a thorough evaluation of safety and biocompatibility has been performed by the scientific community. Nanocelluloses are often discussed as biocompatible and non-toxic. However, cytotoxicity of the structured materials may differ from the cytotoxicity of the suspensions. In their review, De France et al. stated that the evaluation of the cytotoxicity of porous materials before and after processing is essential, although not commonly documented (De France et al. 2017). Key papers on the biocompatibility of nanocellulose structures are thereafter presented. Biocompatibility of CNF films and foams with fibroblast cells (implicated in tissue regeneration and wound healing) was investigated by Alexandrescu et al. (Alexandrescu et al. 2013). Cationic cross-linked cellulose films were used as a scaffold for the assessment of cell attachment and spreading (Courtenay et al. 2018). Scaffold stiffness is modulated to tune cell response. The dermal, oral and ocular toxicity, as well as environmental effect of lignin-coated CNF and CNC materials, were evaluated (Ong et al. 2017). No toxicity was reported.
The influence of the functional groups of nanocellulose has been investigated by Rashad et al. (Rashad et al. (2017). They studied direct and indirect toxicity of TO-CNF and carboxymethylated CNF (CM-CNF) hydrogels. In direct contact with mouse fibroblasts, TO-CNF hydrogels appeared non-toxic; however, direct contact with CM-CNF hydrogels influenced the spreading of cells and their morphology. The same team evaluated the production of inflammatory cytokines by human macrophages in the presence of TO-CNF and CM-CNF scaffolds (Rashad et al. 2019). Both scaffolds demonstrated early mild responses compared to the control group. At day 4, the production of inflammatory cytokines was profiled in subcutaneous tissues of rats and was observed to be lower with CNF scaffolds than with gelatin scaffolds. However, no degradation of the CNF scaffold was observed even after 180 days, resulting in late body reaction response.
A wide range of nanocellulose-based structures can be obtained with controlled and varied properties. Their bio-based and renewable character, non-toxicity and cytocompatibility make them promising materials for biomedical applications. In the next section, their potential use as wound care dressings will be investigated.
