Chitosan is the collective name for a group of fully and partially deacetylated chitins. Their fraction of acetylation FA influences the properties of chitosan, (also described as Degree of Acetylation (DA) The pattern of acetylation (PA) and their degree of polymerization. These parameters influence the physicochemical properties strongly from which the solubility in acidic conditions and interchain aggregation. They influence the biological activities of chitosan and chitosan oligosaccharides. Hence, accurate structural characterization is a key factor in understanding the structure-function relationships of chitosans.
The characterization of a chitosan sample requires the determination of its average DA. Various techniques, in addition to potentiometric titrationRusu-Balaita, 2003, have been proposed, such as InfraredBrugnerotto et al., 2001; Miya et al., 1980; Baxter et al., 1992; Domszy et al.,1985, elemental analysis, an enzymatic reactionPeletier et al., 1990Ultraviolet Muzzarelli & Rochetti, 1985,1H liquid-state NMR Rinaudo et al., 1992 and solid-state 13C NMR.Saito et al., 1987; Raymond et al., 1993; Heux et al., 2000 Those different methods were discussed.Kumirska et al., 2010
The fraction of –NH2 in the polymer (which determines the DA) can be obtained by dissolution of neutral chitosan in the presence of a small excess of HCl, followed by neutralization of the protonated –NH2groups by NaOH, in excess, using pH or conductivity measurements and then followed by titration with HCl to confirm the NH3+ content. These techniques and the analysis of the data obtained have been described.Rusu-Balaita et al., 2003 1H NMR is the most convenient technique for measuring the acetyl content of fully soluble chitosan samples.
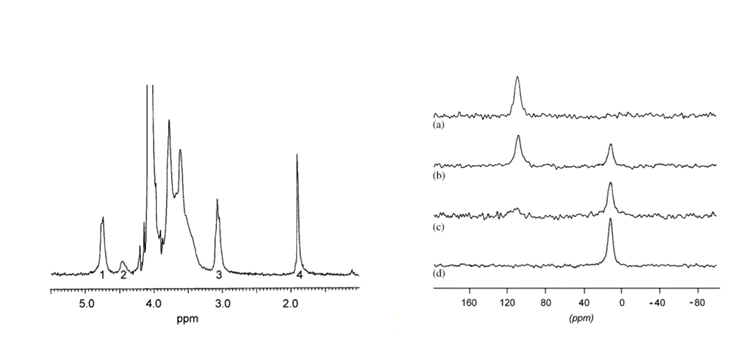
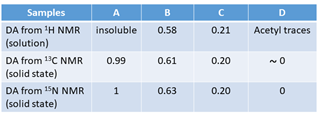
As an example, Table 3 lists the values of DA obtained on four samples : A is an α-chitin, B is homogeneous re-acetylated chitosan and C, D are commercial samples Heux et al., 2000 15N NMR gives only two signals related to the amino group and the N-acetylated group. This technique can be used in the solid-state, whatever the DA. 13C was also compared with 1H NMR and 15N NMR. A good agreement was found over the entire range of DA, irrespective of the state of the sample.
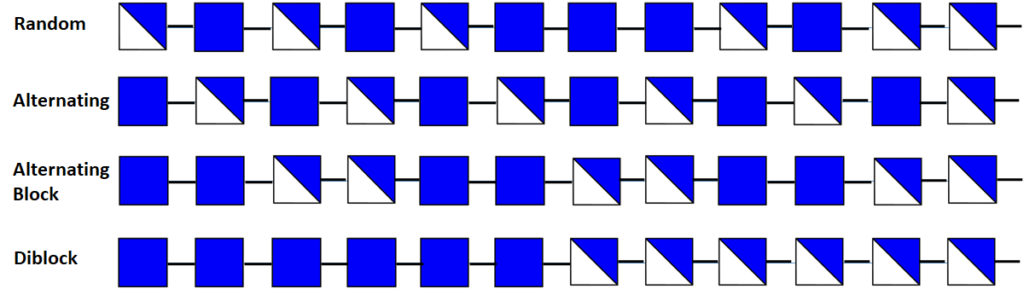
The distribution of acetyl groups along the chain (PA) (random or blockwise) may influence the solubility of the polymer and also the inter-chain interactions due to H-bonds and the hydrophobic character of the acetyl group. This distribution was evaluated from 13C NMR measurements ;Varum et al.,1991; Varum et al., 1991 diad and triad frequencies were determined for homogeneous and heterogeneous chitosan with different values of DA. More recently, 13C NMR is analyzed following Varum et al. but introducing also alternated frequency of the two different types of units.Kumirska et al., 2009; Kumirska et al., 2010; Kumirska et al., 2009 Additionally, mass spectrometry was developed and discussed.Kumirska et al., 2010
Several Infrared techniques are used to assess the acetylation faction. They all require the knowledge of a few reference sample of known FA. The PA can be assessed by the ratio of the intensity of the characteristic band of N-acetylation (which is a measure of the N-acetyl or amine content) to that of the intensity of a band that does not change with different FA value. There exist variations around these methods making use of different absorption band ratios.
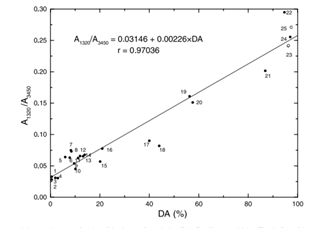
The use of infrared spectroscopy for characterization of the composition of chitin and chitosan covering the entire range of degree of acetylation (DA) and a wide variety of raw materials is examined. The ratio of absorbance bands selected was calibrated using 1H liquid and 13C CP-MAS solid-state NMR as absolute techniques. IR spectra of the structural units of these polymers validated the choice of baselines and characteristic bands. The bands at 1650 and 1320 cm-1were chosen to measure the DA. As an internal reference, the intensities at 3450 and 1420 cm-1 were evaluated. The absorption ratio A1320/A1420 shows superior agreement between the absolute and estimated DA-values.Brugnerotto et al., 2001
Fingerprinting techniques have expanded the possibilities for polysaccharide analysis, typically involving partial depolymerization and yielding mixtures of oligomers, which are subsequently analyzed. When combined with mass spectrometry (MS), these fingerprinting techniques have the advantage of extremely high sensitivity while at the same time reducing the sample amount from the milligram to the micro- or nano-gram rangeWeikert et al., 2017. Fingerprinting techniques require partial depolymerization of the sample, which is achieved using chemical, physical, or enzymatic treatments. Enzymatic depolymerization might be preferred because enzymes often have higher cleavage specificities than the other methods. In the case of chitosan, an enzymatic sequencing approach uses a combination of an exo-β-N-acetylhexosaminidase and an exo-β-glucosaminidase, which removes in an alternative fashion, GlcNAc and GlcN from the non-reducing end of the analytes. A broader range of chitosan hydrolyzing enzymes, particularly chitinases and chitosanases, can be involved. Chitinases cleave the glycosidic linkage between two adjacent GlcNAc units (A/A), and some chitinases may cleave the GlcNAc-GlcN (A/D) or the GlcN-GlcNAc (D/A) linkage. Similarly, chitosanases cleave the glycosidic linkage between two adjacent GlcN units (D/D), and some can cleave A/D or D/A. The more specific enzymes, which selectively cleave only A/A or only D/D, are preferred for fingerprinting. The amounts of the different chitooligosaccharides derived from the enzymatic hydrolysis of chitosan are determined using Ultra-high performance liquid chromatography-electrospray ionization – mass spectrometry (UHPLC-ES-IMS). Data interpretation in the form of Partial Least Squares Regression yields determination of FA.
The PA analysis of chitosan polysaccharides relies on the analysis of dyad frequencies of the C-5 carbon resonances area in the 13C NMR spectra. These studies were performed on chitosan samples derived from chemical preparation methods, including homo- and heterogenous de-N-acetylation and homogeneous N-acetylation. These investigations did not find evidence for a clear non-random PA in any of the samples. As the consequence of a continuous interplay between the results of the analysis of the enzymatic hydrolysis and the nature of these enzymes, new degrading enzymes generating non-random patterns of acetylation can be imagined and produced in a recombinant form. By this token, it is easy to produce enzymatically de-N-acetylated chitosan polymers. By controlling and keeping FA and DP of the polymers constant, differences in functionalities of the polysaccharides could be assessed to the individual differences of their PA.
