8.1. Solubility of Chitin
Chitin occurs naturally partially deacetylated (with a low content of glucosamine units), depending on the sourceMathur & Narang, 1990nevertheless, both α and β forms are insoluble in all the usual solvents, despite natural variations in crystallinity. The insolubility is a major problem that confronts the development of processing and uses of chitin. An important mechanism is a solid-state transformation of β-chitin into α-chitin which occurs by treatment in strong aqueous HCl (over 7M) and washing with water.Saito et al., 1997 In addition, β-chitin is more reactive than the α form, an important property concerning the enzymatic and chemical transformations of chitin.Kurita et al., 1993
Because of the solubility problem, only limited information is available on the physical properties of chitin in solution. The first well-developed studyAustin, 1984, 1975introduced the solubility parameters for chitin in various solvents. This gave rise to the formation of a complex between chitin and LiCl (which is coordinated with the acetyl carbonyl group). The complex is soluble in dimethylacetamide and in N-methyl-2-pyrrolidone. The same solvents and, especially, LiCl/DMAc mixtures, are also solvents for cellulose. Formic, dichloroacetic and trichloroacetic acids for dissolution of chitin chains are also used. Experimental values of parameters K and a relating intrinsic viscosity [η] and molecular weight M for chitin in sever-al solvents according to the well-known Mark–Houwink equation [η] = KMa. are given in Table 4.
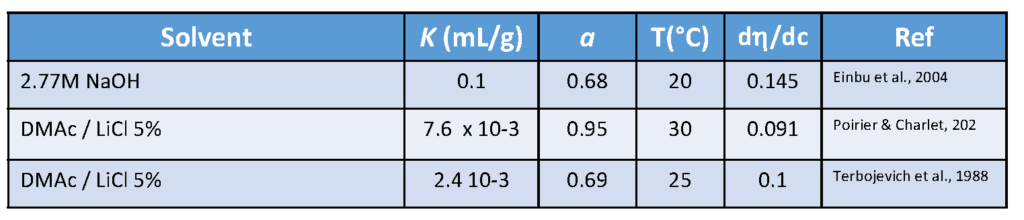
For a long time, the most widely used solvent for chitin was a DMAc/LiCl mixture, though CaCl2 2H2O-saturated methanol was also employed, as well as Hexa-fluoro-isopropyl alcohol and Hexa-fluoro-acetone sesquihydrate.Tamura et al., 2003; Carpozza et al., 1976Concentrated phosphoric acid at room temperature, dissolves chitin.Vincendon et al., 1994 In this solvent, decreases of the viscosity and of the molar mass were observed with time with no change in the degree of acetylation. The use of a fresh saturated solution of lithium thiocyanate was instrumental to record well-resolved NMR spectra at 90 °C.Gagnaire et al., 1982; Vincendon, 1985
A few papers deal with the preparation of alkali chitin by the dissolution of chitin at low temperature in NaOH solution. The chitin is first dispersed in concentrated NaOH and allowed to stand at 25 °C for 3 h or more ; the alkali chitin obtained is dissolved in crushed ice around 0°C. This procedure allowed the authors to cast transparent chitin film with good mechanical properties.Einbu et al., 2004; Sannan et al., 1975; 1976 The resulting chitin is amorphous and, under some conditions, can be dissolved in water, whereas chitosan with a lower degree of acetylation (DA), and ordinary chitin are insoluble. The authors interpreted this phenomenon as related both to the decrease of molecular weight under alkaline conditions and to some deacetylation. They confirmed that to get water solubility, the DA has to be around 50%. Presumably, the acetyl groups must be regularly dispersed along the chain to prevent packing of chains resulting from the disruption of the secondary structure in the strong alkaline medium.Sannan et al., 1976; Kubota & Eguchi, 1997 A study, utilizing techniques such as rheology, turbidimetry, and fluorescence, demonstrated that alkali chitin solubilized in cold (0 °C) aqueous NaOH (16% w/w), according to with the protocol of Sannan et al.Sannan et al., 1975; 1976, forms an LCST solution with a critical temperature around 30 °C.Arguelles-Monal et al., 2003
A chitin gel, obtained from the solution by washing to extract NaOH, was found to be temperature and pH-sensitive.Sannan et al., 1975,1976, Goycoolea et al., 2006 These authors reported the occurrence of a volume phase transition at 21 °C as the result of the influence of temperature on polymer-polymer and polymer–water interactions such as hydrogen bonding and hydrophobic interactions. This transition occurs only within a narrow range of pH (7.3–7.6) and modifies the mechanical shear modulus as a function of oscillating variation in temperature.
Chitin can be dissolved and regenerated from various imidazolium-based ionic liquids such as 1-butyl-3-methylimidazolium acetate and 1-butyl- 3-methylimidazolium chlorideWu et al., 2008 1-allyl-3-methylimidazolium bromidePrasad et al 2009, and 1-ethyl-3-methylimidazolium propionateMundsinger et al., 2015 have been employed. Other Ionic Liquid systems have been investigated. For example, tris(2-hydroxyethyl)methylammonium acetate with added ethylenediamine, could dissolve chitin without heating.Shimo, et al., 2006 Nevertheless, in addition to the nature of the Ionic Liquids, the solubility of chitin seems to be moderate and depends on the molecular weight and on the Degree of Acetylation.Wang et al., 2010Xie et al., 2006 reported that 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) can dissolve pure chitin and chitosan with solubilities of ca. 10 wt% in 5 h at 110 ◦C. Yamazaki et al.(Yamazaki et al., 2009) obtained similar solubilities with 1-allyl-3-methylimidazolium bromide [Amim]Br at 100 ◦C for 24 h.Wu et al., 2008 ‘Native’ chitin could be dis-solved using the acetate salt [C4mim]OAc with 3–7 wt% solubility at 110 ◦C. It is claimed that dissolution of dried shrimp shell in Ionic Liquids allows getting pure chitin while byproducts (such as calcium carbonate) remain undissolved and could be centrifuged out. The following coagulation in a non-solvent (water or methanol) proteins and fatty acids remain in the water-Ionic Liquid mixture after coagulation and are removed during regeneration.Rahman et al., 2009
The rheology of chitin in solution is that of a semi-rigid polysaccharide for which the conformational analysis has been developed in comparison with chitosan. Chitin has been completely dissolved in NaOH/urea aqueous solution at low temperature (5°C) to obtain a transparent solution to determine the persistence length Lp in absence of aggregates. In this solvent, chitin behaves as a worm-like chain with Lp= 30 nm. Fang et al., 2015 The solution must be diluted to avoid the formation of aggregate formation which increases with polymer concentration and temperature. A series of functional chitin-based mate-rials such as hydrogels, aerogels, films, fibers, and microspheres with a homogeneous structure and excellent properties have been obtained.Fang et al., 2015 Several biocompatible chitin-based aerogels, fibers, and hydrogels have been directly constructed.Zhang, 2015 A facile method for the construction of nano-fibrous microspheres from chitin in NaOH/urea aqueous solution through thermally-induced self-assembly was reported for the first time.Zhang 2015
8.2. Solubility of chitosan
When the degree of deacetylation of chitin reaches about 50% (depending on the origin of the polysaccharide), it becomes soluble in aqueous acidic media and is called chitosan. The solubility is a complicated parameter to control : it is related to the DA or FA, the ionic concentration, the pH, the nature of the acid used for protonation, and the distribution of acetyl groups along the chain, as well as the conditions of isolation and drying of the polysaccharide. The intra-chain H bonds involving the hydroxyl groups are also important. The microstructure of the polysaccharide plays a significant role when a fully deacetylated chitin is reacetylated in solution ; the critical value of chitosan DA required to achieve insolubility in acidic media is then greater than 60%. Also, solubility at neutral pH has been reported for chitosan with DA around 50%.Aiba, 1991 A water-soluble form of chitosan at neutral pH was obtained in the presence of glycerol 2-phosphate.Chenite et al., 2000; Chenite et al., 2001; Molinaro et al., 2002; Cho et al., 2005 Stable solutions were obtained at pH 7–7.1 and room temperature, but a gel formed on heating to about 40 °C. The sol-gel transition was partially reversible, and the gelation temperature depended slightly upon experimental conditions.Rinaudo, 2006
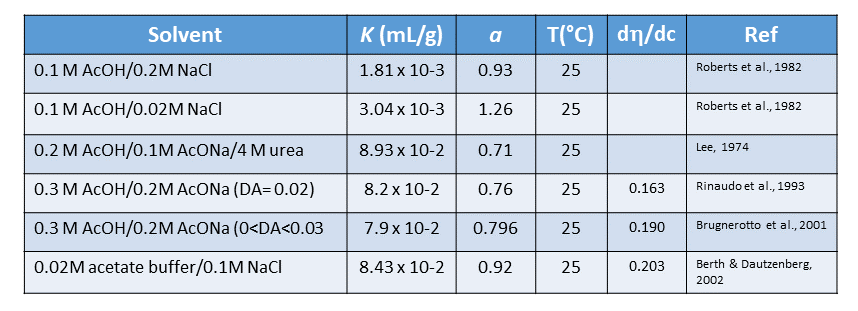
The solution properties of chitosan depend not only on its average DA but also on the distribution of the acetyl groups along the main chain, in addition to the molecular weight. (Kubota & Eguchi, 1997 ;Aiba, 1991 ;Rinaudo & Domard, 1989) The deacetylation is usually performed in the solid-state ; it gives an irregular structure due to the semi-crystalline nature of the initial polymer. Examination of the role of the protonation of chitosan in the presence of acetic acid (Rinaudo et al., 1999) and hydrochloric acid on solubility (Rinaudo et al., 1999) showed that the degree of ionization depends on the pH and the pK of the acid. The solubilization of chitosan with a low DA occurs for an average degree of ionization α of chitosan around 0.5 ; in HCl, α = 0:5 corresponds to a pH of 4.5–5. The solubility is also a function of the ionic concentration. An excess of HCl (1M HCl) creates a salting-out effect allowing the preparation of the chlorhydrate form of chitosan. When the chlorhydrate and the acetate forms of chitosan are isolated, they are directly soluble in water giving an acidic solution. (Rinaudo et al., 1999) Study of these solutions allows the determination of the intrinsic pK (pK0=6 (0.1)) by extrapolation of pK for a degree of protonation α=0. This intrinsic value of the pK agrees with the previous measurements. (Domard, 2000) Thus, chitosan is soluble at pH below 6. The solubility of chitosan is usually tested in acetic acid by dissolving it in 1% or 0.1M acetic acid. The amount of acid needed depends on the quantity of chitosan to be dis-solved. (Rinaudo et al., 1999) The required concentration of pro-ton must be at least equal to that of -NH2 units involved.
The aqueous solution containing LiOH/KOH/urea/H2O in the weight ratio (4.5 wt % LiOH/7 wt % KOH/8 wt % urea) via the freezing-thawing process was used as an alkaline solvent of chitosan (Duan et al., 2015). To prepare the solutions, chitosan powders (with DA=0.11) were dispersed into the alkaline aqueous solvent with stirring for 5 min and then were stored under refrigeration (−30 °C) until completely frozen. The frozen solid was fully thawed and stirred extensively at room temperature. After removing air bubbles by centrifugation at 7000 rpm for 10 min at 5 °C, a transparent chitosan solution with the concentration of 4 wt %. A temperature increase yields a different morphology.