10.1. Complex formation with metals
Chitosan exhibits good complexing ability throughout the involvement of the –NH2 groups along the chain, in specific interactions with metals. Many articles have been devoted to the formation of complexes for the recovery of heavy metals from various wastewaters.Muzarelli, 1973 A mechanism for complex formation with copper at pH>5, was proposedDomard, 1987 (in agreement with X-ray data on chitosan–copper stretched films Ogawa et al., 1984). The mechanism of complex formation with copper in dilute solution was re-examined ; two different complexes were proposed to occur, depending on the pH and copper content.Rhazi et al., 2002
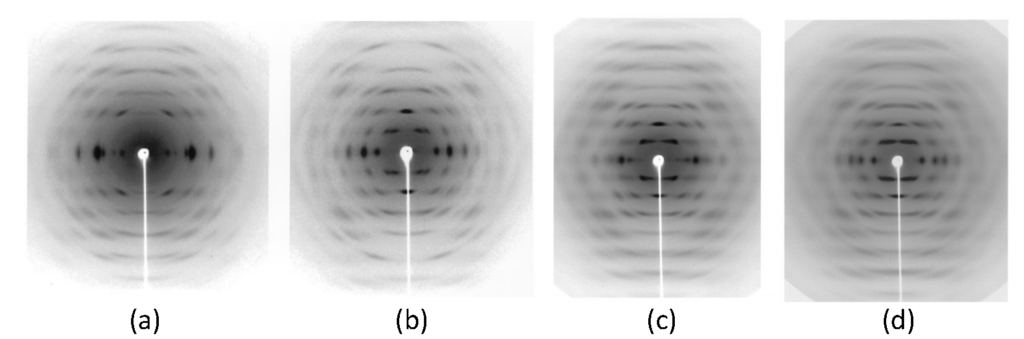
Figure 16. X-ray diffraction pattern of chitosan complexed to (a) HNO3 salt; (b) ZnCl2 salt; CdCl2 salt; and (d) CdSO4 salt. (Okuyama et al., 2000)
This chelation depends on the physical state of chitosan (pow-der, gel, fiber, film). Higher degrees of deacetylation of chitin generates better chelation. Thus, chelation is related to the –NH2 content as well as to their distribution.Kurita, et al., 2002 It is also related to the DP of oligo-chitosans ; the com-plex starts to form, at a degree of polymerization of 46.Rhazi et al., 2002 The two following forms are proposed :
[Cu (-NH2)]2+, 2OH–, H2O and [Cu (-NH2)2]2+, 2 OH–
The first complex is formed at pH between 5 and 5.8, whereas the second forms above pH 5.8 ; the maximum amount of copper fixed is [Cu]/[-NH2] = 0.5 mol/mol. The nature of the cation is critical in the mechanism of interaction. The affinity of chitosan for cations absorbed on film shows selectivity following the order :
Cu2+>>Hg2+>Zn2+>Cd2+>Ni2+>Co2+ Ca2+ ; Eur3+>Nd3+>Cr3+>Pr3+
for divalent and trivalent cations used as their chlorides.

The effect of the nature of the anion was separately demonstratedRhazi et al., 2002; Mitani et al., 1991 : e.g., sulfate increases the fixation on swollen chitosan beads. In another study, chitosan powder was dispersed in a silver nitrate solution or used to fill a column to adsorb mercuric ions from a chloride solution. Peniche-Covas et al., 1992 The conditions for using chitosan (50 mesh particles of chitosan or chemically cross-linked beads of chitosan) play a large role in the adsorption. This is also true for the kinetics of retention.Ruiz et al., 2002; Annachhatre & Chandrachang, 1996 Mixing chitosan powder in 1.5M ferric chloride yields the formation of the complex of chitosan with Fe3+. The solid formed was washed, dried and investigated.Nieto et al., 1992 These authors obtained intra-molecular water-soluble chitosan–Fe(III) complex and deter-mined that one Fe3+ interacts with two chitosan residues, three molecules of water and one chloride ion. The general formula given is :
[Fe(H2O)3(GlcN)2Cl] Cl2.H2O,
where GlcN represents the glucosamine moiety. An aqueous solution of polymer and ferric chloride mixed in stoichiometric proportions yields formation of the complex. One Fe3+ interacts two –NH2 groups and four molecules of oxygen from which at least one water molecule. The remaining N and O are part of the two saccharide units of chitosan (Fe3+ are Hexa or Penta coordinated).Bhatia & Ravi, 2000
Chitosan extracted from tendon was immersed in solutions of various salts of transition metals and further submitted to X-ray investigation.Ogawa et al., 1992 The ratio of glucosamine to copper (II) was 2:1. The crystal structure of CuCl2/chitosan was different from that in complexes formed with other salts. Several derivatives of chitosan have been prepared in view of enhancing the formation of complexes.Gomez-Guillen et al., 1992; Chiessi et al., 1993; Hall & Yalpani, 1984; Muzzarelli et al., 1982a,b; Muzzarelli, 1989 In one study, the same order of ionic selectivity for divalent cations, as given above,Rhazi et al., 2002was found by calorimetric measurements with N-carboxymethyl chitosan.Muzzarelli, 1989
Chitosan, as a polyelectrolyte, forms electrostatic complexes under acidic conditions. Two different types of complexes are considered : electrostatic complexes with an oppositely charged surfactant (SPEC) and polyelectrolyte complexes (PEC).
10.2. Complexes with surfactants.
The general behavior of polyelectrolytes is demonstrated with chitosan and sodium dodecyl sulfate (SDS). An electrostatic complex forms in the presence of a low DA chitosan involving cooperative stacking of surfactant alkyl chains. The association forms a micellar system that precipitates out. The addition of tiny amounts of surfactant generates interesting interfacial properties. Surface tension measurements detect a critical aggregation concentration (c.a.c.) around 100-fold smaller than the c.m.c. of the surfactant alone.Desbrieres et al., 1997; Babak et al., 2002 The cooperativity of the observed interaction depends directly on the charge density of the chitosan (in fact, it depends on the distance between two adjacent ionic sites) as shown for carboxymethyl chitin in the presence of tetra-decyl-tri-methylammonium bromide (TTAB).Desbrieres & Rinaudo, 1999
There is the formation of a capsule when a chitosan solution is dropped into an SDS surfactant solution ; a chitosan gel layer (characterized by an ordered nanostructure) cross-linked by charged surfactant micelles is formed in the interfacial film.Babak et al., 2000abcSuch a structure can encapsulate enzymes. Babak et al., 2001 This type of electrostatic complex has been examined by calorimetry. The strong affinity and its dependence on the excess of external salt confirm the electro-static mechanism.Prado et al., 2004; Thongngam & McClements, 2004; Thongngam & McClements, 2005 This electrostatic interaction can be compared with covalent analogs obtained by graft-ing alkyl chains on a chitosan backbone (see below for a description of these derivatives). The interfacial properties of the chitosan-derived polymer surfactant display a relatively low surface tension activity but interesting bulk properties. The role of sulfated N-acyl chitosan (S–Cn–Chitosan) in a lipid mem-brane compared with that of SDS. This SDS dissociates the membrane, whereas the polymer stabilizes the membrane, and even increases its rigidity, suggesting low toxicity in bio-organisms. In solution, when the alkyl chain in S–Cn–chitosan is longer than ten units, the polymers form more stable micelles than those formed by the same alkyl chain surfactant alone.Nonaka et al., 2002 Interactions of this kind are relevant to the field of food chemistry, involving specific interactions of chitosans with phospholipids and bile acids.Thongngam & McClements 2005
10.3. Complexes with oppositely charged polymers (macro-molecules, polyanions, DNA)
Mixing oppositely charged polyelectrolytes results in the formation of a polyelectrolyte complex (PEC) based on electrostatic interactions depending upon pH and external salt concentration.Kabanov, 2003; Kabanov & Zezin, 1984 Developments of new biomaterials production and novel biomedical applications use electrostatic complexes involving natural biopolymersJeong et al., 2017; Volodkin et al., 2007 Strong electrostatic interactions between oppositely charged systems takes place at the interface. They can stabilize or destabilize liposomes for drug release Volodkin et al., 2007; ial et al., 2005 or for the formation of lipo-plexes especially with DNASerikawa et al., 2000; Stephan et al., 1996; Bochicchio et al., 2015 or to stabilize a colloidal dispersion.Domard et al., 1989; Pfefferkorn, 1995 It has been demonstrated that liposomes are stabilized by chitosan adsorption against osmotic or pH shocks and that chitosan is adsorbed flat on the surface.F. Quemeneur, M. Rinaudo, G. Maret & B. Pepin-Donat, Decoration of lipid vesicles by polyelectrolytes: mechanism and structure, Soft Matter, 6, 2010, 4471-4481)
Layer by layer formation between oppositely charged polyelectrolytes is an important development of polyelectrolyte complexes. It was applied to form capsules by deposition on liposomes and their stabilizationFukui & Fujimoti, 2009; Angelini et al., 2008, to stabilize the biological activity of peptide hormones or to coat blood vessel using chitosan and hyaluronan.
Literature cites many electrostatic PEC between chitosan and synthetic or natural charged polymers : e.g. polyacrylic acid, sodium salt (PAA), carboxy-methyl-cellulose Peniche & Arguelles-Monal, 2001; Arguelles-Monal & Peniche,1988, xanthan, carrageenan, alginate (extracted from brown algae), pectin, heparin, hyaluronan (HA)Rusu-Balaita et al., 2003; Vasiliu et al., 2005 sulfated cellulose, dextran sulfate, N-acylated chitosan/ chondroitin sulfate.Kubota & Kikuchi, 1998; Goycoolea et al., 2000The electrostatic interaction has been discussed in relation to the stiffness of the backbone and nature of the ionic groups involved. Especially with alginate or HA, a pH-dependent com-plex occurs, whose stability depends on the ionic strength. The complex formation was investigated in dilute solution by potentiometry following changes in pH and conductivity to determine the fraction of ion pairs (–COO–+NH3–) formed, depending on the experimental conditions. Rusu-Balaita et al., 2003; Arguelles-Monal et al., 2000 The interaction between chitosan and alginate gives an electrostatic complex which, so far, has been used mostly for biological applications.
The main applications of these electrostatic complexes are anti-thrombogenic materials, controlled release systems, encapsulation of drugs, immobilization of enzymes and cells, and gene carriers. Among the examples, are the applications of alginate/chitosan complexes. One aspect of these complexes is dealt with the preparation, layer-by-layer (successively, one layer of polyanion–one layer of polycation), of polyelectrolyte capsules or films based on charged biocompatible polysaccharides or chitosan/synthetic PEC.Vasiliu et al., 2005; Zang et al., 2005; Berth et al., 2002 In the case of chitosan Zang et al., 2005, PAA is used to form the capsules, then the chitosan is cross-linked, and the PAA is re-dissolved. Such chitosan capsules are more stable than in the absence of chemical crosslink-ing ; they are pH-sensitive, swell at low pH, and shrink at high pH. Calcium alginate gel-stabilized by complexation with galactosylated chitosan (a water-soluble derivative) yields a porous gel (sponges).Chung et al., 2002 Dropwise addition of Na–alginate to chitosan–CaCl2 solution produces a complex in the form of beads. These beads differ from Ca-alginate beads in exhibiting maximum swelling at pH 9Lee et al., 1997 Oligo-chitosans, low molecular weight chitosans, were also complexed with alginates to form capsules with controlled permeability.Bartkowiak & Hungeler, 1999; 2000; Bartkowiak et al., 2000
Chitosan characteristics are most important in complex formation since they control the condensation and their stability. Many characteristics of chitosan have been previously discussed in the literature.Rinaudo, 2006 and summarized as follows : (1) chitosan molar masses MW (partial depolymerization with sodium nitrite generates samples with different MW ).MacLaughlin et al., 1998 (ii) chitosan degrees of acetylation (DA usually between 0 and 0.3 to be soluble in acidic conditions) (iii) their acetyl groups distribution along the chains (usually not examined in complex formation).
Several articles deal with the influence of MW and DA of chitosan on complex formation with DNA.Strand et al., 2005; Buschmann et al., 2013; Bordi et al., 2014; Ma et al., 2009; Amaduzzi Alatorre-Meda et al., 2009; Lavertu et al., 2006In these works, the main techniques used are electrophoretic mobility (and zeta potential), Dynamic Light Scattering (DLS), Atomic Force Microscopy (AFM), potentiometry or microcalorimetry. Since chitosan is a weak base with a pK0=6.5, pH is an essential factor to control the degree of protonation of the amino groups ; the positive fraction of charge (-NH3+) being the factor which controls the complex formation with the highly negatively charged DNA.Strand et al., 2005; Sato et al., 2001 The higher the degree of chitosan protonation, the stronger is the stability of complex formed with DNA and the condensation of the complex.Strand et al. 2005 A minimum of 6 to 9 monomeric units is necessary to complex DNA. However, the stability of the complex tends to be weak, dissociating at pH> 6.5 or more than salt.Strand et al., 2005 The stability of the complex depends on pH, N+/P- charge ratio, and salt concentration in vitro.
The most important parameter after chitosan characteristics is the ratio N/P (or chitosan units/phosphate units). For progressive additions of chitosan at a pH lower than 6.5 to a dilute DNA solution, N+/P- increases while the complex forms. The complex is negatively charged, up to an isoelectric point followed by the charge inversion. The N+/P- charge ratio at null charge is usually found around the charge stoichiometry when only the protonated fraction is considered.Bravo-Anaya et al., 2016 For gene delivery, it is important to use a positive com-plex (N+/P- >1) in the nanometric range of particle diameters able to interact with the negatively charged cell membrane. This is a requirement to the entrance in the cell through endocytosis and pinocytosis to allow transfection.Mao et al., 2001; Strand et al., 2005; Buschmann et al., 2013; Amaduzzi et al., 2014; Lavertu et al., 2006 It was claimed that N/P molar ratio=3 gives the highest transfection activity in serum using an MW=70 000 chitosan. Compared with other polycations often proposed for gene therapy, chitosan has lower toxicity than polylysine. After 96 hours, this polycation was ten times more efficient than PEI.Erbacher et al., 1998
Another important and delicate point is the evaluation of the transfection efficiency of chitosan/DNA systems in vivo. The stability of the complex depends on several factors such as chitosan characteristics (DA and MW), local pH and salt composition, charge ratio of chitosan to DNA (N+/P-) playing on complex stability. It also depends on the cell type, nanoparticle size, enzyme and protein interactions, and interactions with membranes. It was reported that the transfection efficiency at pH 6.5 was higher than at pH 7.4.Nimesh et al., 2010 At pH=8, the complex was fairly insoluble and did not penetrate the membrane.Erbacher et al., 1998 Nevertheless, the transfection efficiency based on DNA/chitosan complex is not yet fully understood. The control of the different steps of the mechanistic pathway for gene transfection is required. It includes the collapse of extended DNA chains into compact nanometric particles. This process, known as DNA condensation, has received considerable attention for the production of gene delivery vehicles V. Vijayanathan et al., 2002, and for this step, chitosan is a good and adequate candidate. Mao et al., 2001Then, the positive particles of DNA compacted by polycations interact with the anionic proteoglycans at the cell surface and are transported by endocytosis. The cationic agents have a buffering capacity in the endosomal pH range (pH 4.5 to 7.5) inhibiting the degradation of DNA by lysosomal enzymes.Buschmann et al., 2013; Richard et al., 2013 The mechanism was fully elucidated for PEI/DNA complexes.Akinc, et al., 2005; Benjaminsen et al., 2013; Neuberg & Kichler, 2014
Potentiometry demonstrated that the buffering capacity, or proton sponge effect, was larger for chitosan than for PEI for the same number of ionic sites.Richard et al., 2013. The presence of an excess of free chitosan increased the osmotic pres-sure and destabilized the endosome. This releases DNA complex migrating into the nucleus where it can decondense after separation from the cationic delivery vehicle and can regulate gene expressionRichardson et al., 1999; Buschmann et al., 2013; Richard et al., 2013; Thibault et al., 2011; Ma, et al., 2010 a,b. In fact, the last limiting step is the un-packaging of DNA from the complex after its localization in the nucleus. Labeled polylysine having different molecular weights that the complex formed dissociates more rapidly with lower molecular weight both in vitro and in vivo.Howard et al., 2006
The question of the stability of the complex in vivo is important but not yet solved : higher stability hampers the transfection efficiency.Koping-Hoggard et al., 2001; Alatorre-Meda et al., 2001 There is a fine balance between extracellular DNA protection (better with high MW and lower DA) and ability of efficient intracellular unpacking (better with low MW) in order to get a large level of transfection.Buschmann et al., 2013; Luo & Saltzman, 2000; Liu et al., 2005; Ma et al., 2009; Lavertu et al., 2006 In a previous work, DNA-chitosan complex stoichiometry, net charge, dimensions, conformation, and thermal stability were determined and discussedBravo-Abaya et al., 2016 The isoelectric point of DNA/chitosan complexes is directly related to the protonation degree of chitosan. The electrostatic interactions between DNA and chitosan are the main phenomena taking place in the solution up to the stoichiometric charge ratio N+/P-=1. This work was completed using DNA concentration in the dilute regime, i.e., around 10 times slower than the average value of the overlap concentration C* (0.23 mg/mL)Bravo-Anaya et al., 2016 to establish the mechanism of chitosan /DNA interaction in relation with the composition or ratio N/P since this point has been rarely covered. The ionic interaction between the negative phosphate sites and the positive -NH3+ from chitosan is essential for complex formation. The influence of chitosan protonation in DNA/complexes stability, as well as chitosan DA (Figure 19), was proposed through the analyses of several physical-chemical and biophysical techniques, i.e., UV-Vis and DLS measurements, and by gel electrophoresis assays.
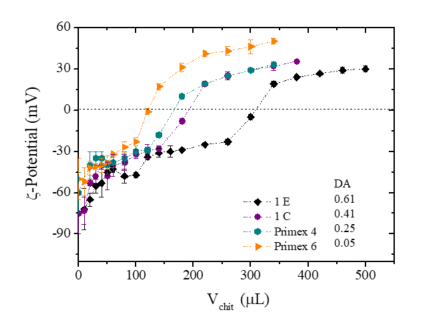
Chitosan samples were prepared in presence of slight excess of HCl and at a concentration of 1 mg/mL Two hypotheses may be suggested for complex formation : i) the complexation occurs randomly in solution, i.e chitosan associated on each DNA chains in the ionic ratio N+/P- ; ii) cooperative interactions with positively charged chitosan saturate a DNA chain, and the complementary DNA fraction remains free of chitosan as long as N+/P-< 1. A remaining question concerns the case when N+/P- > 1 : when the complex becomes positively charged. Is there an excess of chitosan fixed on the complex to control this charge inversion ? Gel electrophoresis was used to demonstrate that the fraction of fixed DNA equals the number of positive chitosan charges added (Figure 20)

This means that complex forms by a cooperative electrostatic interaction. In the same work, using fluorescent chitosan it was shown that no free chitosan is left when N+/P- 1. In excess of chitosan, a small fraction is additionally fixed on the complex to turn the net charge to positive up to R=N/P =3. The excess of chitosan is essential in the buffering effect, as demonstrated by potentiometry (Figure 21)
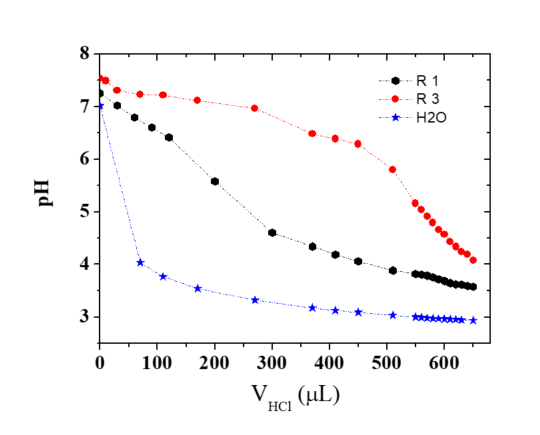
The pH remains nearly constant when HCl is added to the system corresponding to R=3 i.e. in the presence of free chitosan.
