Human epithelial cells are found at the forefront of human anatomy separate the body and its cavities from the exterior environment. Their glycocalyx nanolayer comprises glycoconjugates: glycoproteins and glycolipids, which present their carbohydrate portion to the extracellular environment. The role of the glycocalyx and its actors is to sense and communicate with their environment in different ways. For example, epithelial cells are the gatekeepers of the body compartments and, as such, need to communicate to establish a stable cellular tissue. This endothelial tissue assembly is ensured by glycocalyx-mediated communication. (Reitsma et al., 2007) Another example of this communication is how glycoconjugates mediate immune self-recognition, allowing the immune system to discern between own and foreign cells and act accordingly. Finally, the glycocalyx can be a biomarker of diseased states like cancer.(Mereiter et al., 2019; Pinho et al., 2015) Theoretically, the structural versatility of glycans allows them to hold an unfathomably large quantity of information. In reality, this information is filtered through physical and biological constraints, resulting in the glycan structures observed in living organisms. The resulting information held by these glycan structures remains vast: the ‘sugar code’ is considered the third alphabet of life, employing monosaccharides as letters in parallel to nucleobases and amino acids.(Gabius, 2018; Kaltner et al., 2019; Solís et al., 2015)
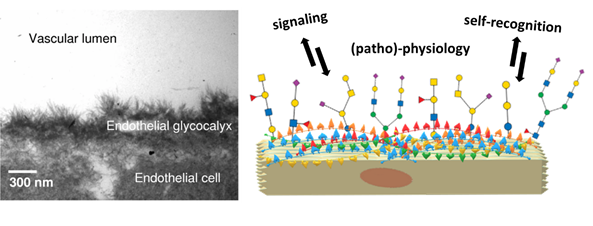
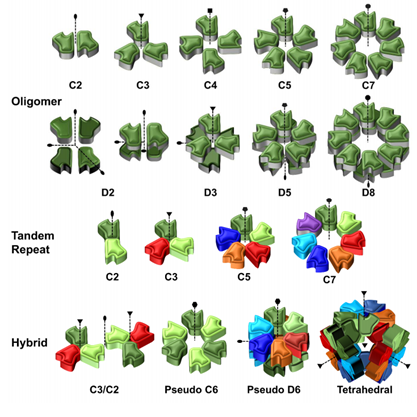
Naturally, for every glycan presented by the glycocalyx as a ‘message’ to its environment, another biomolecule plays the complementary role of ‘reader’. Lectins are ubiquitous carbohydrate-binding proteins, key recognition agents for intercellular interactions at the extracellular matrix. Lectins have been studied extensively, owing to their role and potential for deciphering the sugar code and providing valuable knowledge over its significance on biological processes.(Sharon et al., 2004; Solís et al., 2015) Generally having a weak millimolar affinity for the monosaccharide version of their ligand, lectins compensate by establishing multivalent interactions mediated by the presence of several binding sites. Indeed, lectins often present elements of structural symmetry: β-propellers, β-trefoils and β-sandwiches in homo-multimeric assemblies are not uncommon. As lectins typically rely on multivalent interactions, they present their binding sites on the same face of the carbohydrate recognition domain (CRD). The prototypical lectin presents many equivalent or quasi-equivalent binding sites on one of its faces, around a symmetry axis, as seen in Figure 3. Although this seems to imply that lectins have low structural diversity, the opposite is true: lectins hold the structural diversity to match the sugar code. Indeed, the richness of specificity and topology observed in lectin scaffolds have made them exciting tools for generating engineered ‘neolectins’ with applications in diagnostics, therapy and material science, among others.(Notova et al., 2020; Ribeiro et al., 2018) Developed in recent years, UniLectin3D is a valuable database for exploring and comparing lectins and scaffolds: it curates lectins by structural features and carbohydrate specificity and even species, highlighting that lectins are ubiquitous. (Bonnardel et al., 2019)
Although lectins do not exclusively mediate intercellular communication, these proteins are notably represented in the interactions between humans and microbes. As mentioned earlier, a prerequisite to attempting AAT is a thorough understanding of microbial virulence factors and their targets in the host/pathogen interface. Carbohydrate-binding molecules (lectins, toxins, adhesins) from bacteria, viruses and even parasites are famously known virulence factors. On the one hand, adhesins are found atop bacterial extracellular organelles – fimbriae and mediate the adhesion of the whole bacterium to any surface that exposes the corresponding carbohydrate epitope. For example, FimH is an extensively studied adhesin that allows Escherichia coli’s fimbriae to adhere to mannosylated residues on human epithelial cells, thus facilitating urinary tract infection (UTI). Recently, mechanical studies performed by atom force microscopy (AFM) have characterized the interactions of FimH and other adhesins as ‘catch bonds’: interactions that get stronger under mechanical tension. (Viela et al., 2020) The mechanical strength observed supplements another characteristic of these virulent interactions: whereas animal and plant lectins usually have low affinity for their targets, microbial lectins and adhesins present sub-micromolar or stronger affinities. (Imberty et al., 2005)
On the other hand, toxins and lectins are, contrary to adhesins, soluble. Toxins are proteins that usually feature different sub-units. The pathogen releases them to recognize epitopes on the surface of target cells, which is mediated by a first sub-unit. Upon binding, toxins are internalized, and their second sub-unit enacts a toxic effect, often leading to cell death. A classic example of such toxins is seen in Figure 4: the AB5 toxin family. AB5 toxins featured in organisms such as E. coli and Bordella pertussis present a cytotoxic ADP-ribosyltransferase (A) domain linked to five (B5) lectin subunits with the capacity to recognize endothelial surfaces.(Imberty et al., 2005; Merritt et al., 1995) Finally, many soluble lectins do not fill the role of either adhesin or toxin. These agents often present specificity to epitopes located at the glycocalyx but are not reduced to these targets. Lectins are versatile and can fill complex roles related to quorum sensing, biofilm formation and even cooperativity across different species of pathogens.
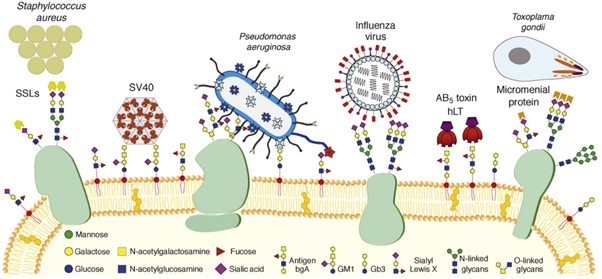
The list of pathogens using lectins for adhesion, infection and toxicity is long: E. coli, Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholera, Clostridium tetani, Influenza viruses, etc. (Imberty et al., 2008). As transpires from Figure 4, many illnesses and pathologies rely on lectins in their initial stages, cementing the idea that AAT would be beneficial to counter MDR pathogens. (Poole et al., 2018) Moreover, lectins have a role in establishing and holding biofilms together, thus boosting resistance to antibiotics. Biofilms occur when the bacterial and fungal cells adhere to a surface and each other to form an extracellular matrix. For pathogenic bacteria, the advantages of forming biofilm are many: stability for growth, change into an infection-adapted phenotype, elasticity against physical forces and, more importantly, resilience against host immune factors and antibiotics. (Hall-Stoodley et al., 2004) Interestingly for AAT, the knock-out or inhibition of biofilm-mediating lectins has led to disruption of biofilm integrity.(Diggle et al., 2006; Inhulsen et al., 2012; Tielker et al., 2005)
As a result, bacterial lectins are twice-verified targets for AAT: antagonizing all pathogenic lectins would certainly be therapeutically advantageous in the context of early infection. However, every project targeting lectins must be unique: most carbohydrate/lectin interactions are specific. Indeed, lectins are as diverse as carbohydrate structures are. Nevertheless, trends do exist in the context of microbial virulence factors and infections.
Among the typical targets for lectins, the role of histo-blood group oligosaccharides in microbial infections is undeniable. (Heggelund et al., 2017) Human oligosaccharides are tightly bound to infection, to the point that evolutionary strategies have developed around them. A clear example of this can be drawn from the staple of mammalian biology: breastfeeding. High concentrations of oligosaccharides occur in the milk of humans and other mammals: these are HMOs (human milk oligosaccharides).
Interestingly, these HMOs present the same epitopes usually recognized by virulence factors. Studies analyzing the influence of HMOs in pathogenicity showed better outcomes for breastfed infants. (Sharon, 2006) It means that mammals confer a true anti-adhesion therapy to the next generation by the mere action of breastfeeding. Regarding the epithelial glycocalyx, the histo-blood group oligosaccharides present large yet well-defined epitopes for lectins to recognize, which explains the high diversity of microbial lectins and the high specificity for their targets.
Some pathogens take the unorthodox approach and display carbohydrates that human lectins can recognize as an alternative to microbial lectins. By high-jacking human bio-machinery, they can infect and enter the human cell in question in the case of viruses. Among the pathogens using this strategy is the well-known HIV: it targets Langerin and the Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN). This receptor belongs to the immune system and can recognize mannosylated glycans characteristic of invasive pathogens such as Ebola, Hepatitis C and HIV. Dendritic cells can travel to lymph nodes and elicit immune responses by binding to these viruses. However, HIV uses this dynamic to propagate and find its way to lymph nodes. Another family of pathogens that have become relevant in recent times uses a similar process: coronaviruses. Indeed, recent studies have confirmed the ability of SARS-CoV-2 to use its spike glycoprotein to target human lectin DC-SIGN and others. (Chiodo et al., 2020) The virus enters human cells thanks to its spike protein and human angiotensin-converting enzyme (ACE2). (Letko et al., 2020) It remains to be seen whether the carbohydrate/lectin interactions discovered are also relevant for adhesion and infection.
Returning to AAT, we mentioned the concept of designing therapeutic agents to mimic and compete against epitopes targeted by virulence factors. Applied to virulent lectins, this translates into designing carbohydrate ligands that can compete against the human oligosaccharides by efficiently binding to the lectins, effectively impeding microbial adhesion, as schematized in Figure 5.
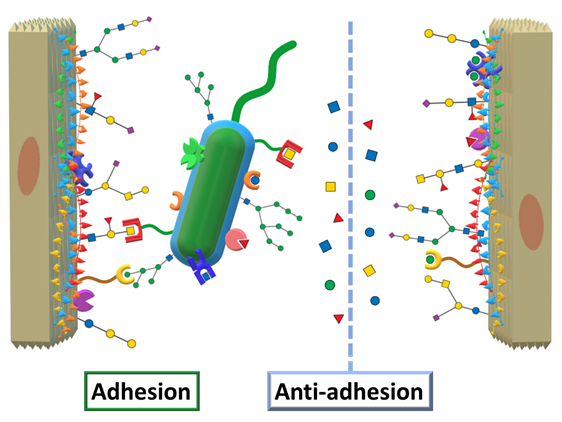
.
