The problem of antimicrobial resistance (AMR)
Many barriers have been met and overcome across the history of the human race and its progress. One of such, and particularly significant, is humans’ fight against pathogenic microorganisms. Very relevant to current times, pathogenic viruses can rise to become global threats, but so can bacteria. Be it the bubonic plague, tuberculosis, cholera, or others. These names still resonate, echoes of times in which the battle against pathogens was lost. In such times, a unicellular organism could singlehandedly decimate a percentage of the human population: for example, tuberculosis peaked in the XIXth century and is estimated to, now, have killed 14% of humanity (all humans that had ever lived to that point), making it the deadliest bacterial infection in history, so far. In 2019, it still managed to infect 10 million and kill 1.4 million people. (WHO, 2020) Adding to this, bacteria have acted in conjunction with pathogenic viruses, for example, during the early XXth century, when the influenza pandemic later called ‘the Spanish Flu’ left millions vulnerable to opportunistic bacterial pneumonia. This pandemic decimated 5% of the world’s population at the time.
The aforementioned dark times came to an end in relatively recent times: as the XIXth century gave its way to the XXth, the rapid development and introduction of many vaccines gave a prophylactic means to fight infectious diseases. More importantly, Alexander Flemming’s chance encounter with penicillin in 1928 paved the way for the direct fight against bacterial infections with antibiotics. Penicillin’s widespread use started in the 1940s during World War 2 and was followed by the ‘Golden Age’ of antibiotics (1950-70s), humanity’s highest point in the fight against microbes. However, by 1955, antimicrobial resistance (AMR) to penicillin was a fact only twelve years after the start of its extensive use, as Flemming himself had predicted. Thus, AMR loomed large over modern medicine and scientists, who kept finding new antibiotics, hoping to keep ahead in the race between humans and AMR pathogens (see Table 1). (Bushak, 2016)
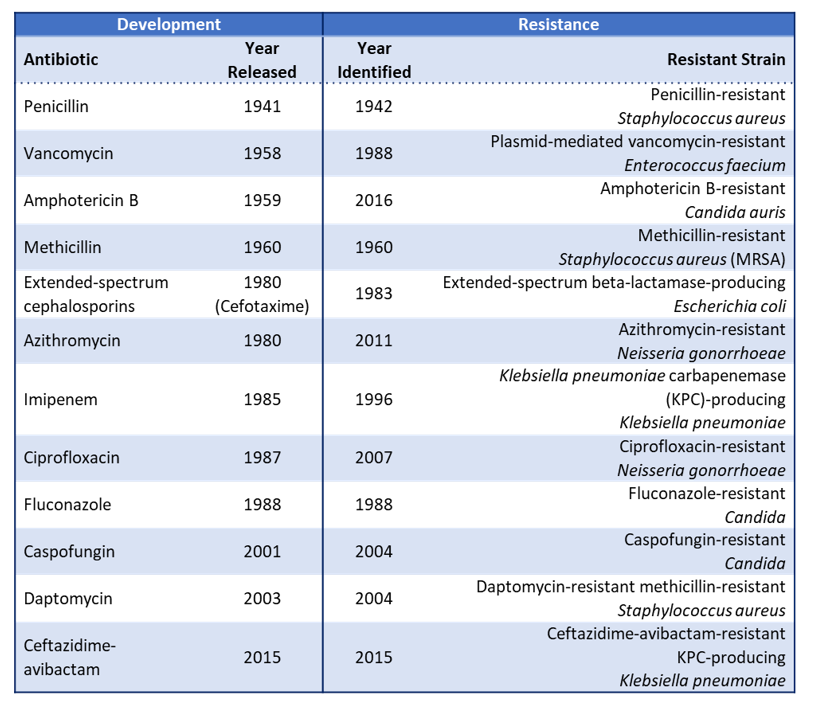
At the beginning of the XXIst century, there is no denying it: we are losing the antibiotics race. As seen in Table 1, the most recently discovered antibiotics (Daptomycin in 2003 and Ceftazidime-avibactam in 2015) lasted only one year before resistance appeared and was documented. (US-CDC, 2019) Names such as MRSA (Methicillin-resistant Staphylococcus aureus) have reached the general public, and names such as ‘superbugs’ have been coined for MDR and PDR (Multidrug- and Pandrug-resistant) bacteria.
Cases of patients infected with superbugs resistant to ‘last-resort’ antibiotics such as colistin already surfaced in 2016. (McGann et al., 2016) These pathogens, resistant to most existing therapies, are especially threatening to hospitalized patients who present risk factors. Risk factors include medical conditions such as cancer, diabetes and immunosuppression, for example, due to chemotherapy. Additionally, immunodeficiency due to either physiological stress (for example, skin damage or malnutrition) or old age can render a patient prey to these pathogens, in what is called an ‘opportunistic’ infection. (Appelgren et al., 2001; Ozer et al., 2010; van Duin et al., 2016) It is evident that the mere presence of these pathogens in medical environments could quickly turn into a worst-case scenario: fragile patients threatened by untreatable bacterial infections. Indeed, MDR microorganisms already represent the leading cause of hospital-acquired infection (HAI) death.(Cassini et al., 2019; van Duin et al., 2016)
Undeniably, HAIs by resistant pathogens can grow into a bigger problem, to the point of reversing years of advances in modern medicine. This issue is illustrated by the situation of patients afflicted with cystic fibrosis (CF). CF is a well-studied genetic disease caused by a cystic fibrosis transmembrane conductance regulator (CFTR) mutation. This dysfunction results in thick mucus accumulating in different organs. Its chief consequence is progressive respiratory problems and increased susceptibility to lung inflammation and infections. Although no definitive cure exists, regular advances in modern medicine have enabled specialized treatment and care for CF patients, improving their quality of life. In terms of life expectancy, children born with CF in 2021 are expected to live 20 years more than the previous generation of patients.(O’Sullivan et al., 2009). Despite this, the leading cause for morbidity and mortality (at least 80%) in this population are bacterial respiratory infections. Indeed, the thick mucus characteristic of CF translates into a reduced capacity for airway cleansing, making the lungs an ideal breeding ground for opportunistic pathogens.(Ciofu et al., 2013) Due to this, CF is considered a high-risk factor in HAIs. It is the main responsible of mortality among genetic diseases in the Caucasian population. Similar to antibiotics, CF patients are losing the battle against MDR pathogens.
Proportional to what is becoming one of the main challenges of the XXIst century, a coordinated response against AMR has been erected at the highest levels: the World Health Organization (WHO), the European Commission, and the United States CDC (Centers for Disease Control and Prevention) all have action plans to implement against the rise of MDR pathogens.(EU-Comission, 2017; US-CDC, 2019; WHO, 2015) These plans provide solid advice on reducing resistance by better handling of antibiotics and highlight the necessity for alternatives in this fight. Therapies involving vaccines, antibodies and bacteriophages are some of the alternatives presented. Another alternative to antibiotics, less conventional but more relevant to this communication, is anti-adhesion therapy.
