As mentioned earlier, lectins are key actors in cell adhesion leading to infection and have proven to be interesting anti-adhesion and combination therapy targets. A prime example of these notions is how inhibiting the soluble lectins of P. aeruginosa with drug-like glycomimetics has led to biofilm disruption and enhanced susceptibility to antibiotics. (Sommer et al., 2018)
Connecting the dots between B. cenocepacia and P. aeruginosa is simple: both hold the same characteristics as MDR opportunistic pathogens, target the same populations, are considered critical lung pathogens, and have been extensively studied through the lens of CF-related research. Moreover, both have similar bio-machinery to establish chronic infection: they rely on quorum sensing, adhesion, phenotypic adaptation, biofilm formation and resistance to therapy. Therefore, screening the genome of B. cenocepacia and other BBC strains for putative lectins using P. aeruginosa’s heavily studied lectins as a template can be considered a reasonable venture. The search thus conducted identified four homologs of lecB on B. cenocepacia strain J2315. (Lameignere et al., 2008) The homologs were called BC2L(-A, -B, -C and -D). There are three genes in chromosome 2, coding for putative lectins A to C, and a final gene on chromosome 3, coding for putative lectin BC2L-D. Although a frameshift invalidated the gene coding for BC2L-D, it was valid in other strains.
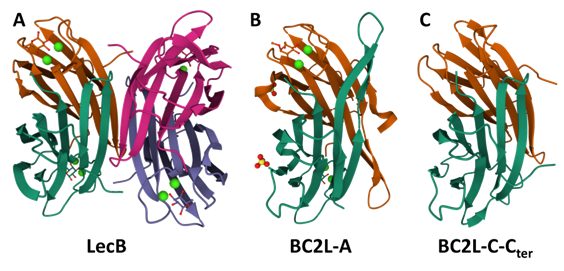
The study of these lecB-like lectin families started by BC2L-A, whose original name ‘BclA’ was aptly changed to avoid redundancy with other protein names such as ‘BclA’ from Bacillus anthracis and the heavily studied ‘Bcl-2’ family of apoptosis regulators involved in cancer research. (Cory et al., 2002) Leading to BC2L-A, the lecB-like gene bclA occurred on other six Burkholderia strains, well-conserved and maintaining 32% similarity with lecB. (Lameignere et al., 2008) It coded for 129 residues, longer than LecB, mainly through insertion in a non-functional region and an elongated N-terminus. Nevertheless, BC2L-A was successfully expressed in native form from B. cenocepacia strain J2315, and later cloned in E. coli and produced in recombinant form, showing the expected LecB-like calcium-mediated specificity for mannoside saccharides.
Indeed, LecB is a fucose-binding lectin that binds mannosides and requires two calcium Ca2+ ions for carbohydrate binding. Interestingly, BC2L-A shows exclusive specificity for mannosides. It is due to a difference in their sequence: a specificity loop formed by residues 22-24 in LecB features two serine residues (22 & 23), which are replaced by alanine (29 & 30) in BC2L-A, thus allowing rationalization for the specificity. Nevertheless, both lectins have an unusually strong affinity for their respective ligands compared to usual monosaccharide/lectin interactions.(Lameignere et al., 2010; Sabin et al., 2006) The structural and functional study of BC2L-A went on to provide crystal structures, extensive probing against mannosides and even successful inhibition with mannoside glycomimetics.(Beshr et al., 2016; Csavas et al., 2017; Lameignere et al., 2008; Lameignere et al., 2010; Pifferi et al., 2017) In these studies, the structural similarities and differences between LecB and BC2L-A were detailed: the homotetrameric form of LecB is inaccessible to BC2L-A, which instead remains a homodimer (see Figure 10). Additionally, the potential of glycomimetics as antagonists of BC2L-A was proven through structural and biophysical evaluation, and BC2L-A proved to be a valuable model to optimize a multivalent glycomimetic design. (Reynolds et al., 2013)
A report of utmost relevance described interactions of BC2L-A with epitopes obtained from bacterial lipopolysaccharides (LPS). (Marchetti et al., 2012) LPS are structural staples of the Gram-negative outer membrane and cover most of the bacterium’s surface. (Bernardi et al., 2013) This could mean that the likely biological function of BC2L-A is to mediate cell-cell adhesion between bacterial cells. Finally, fluorescent-tagged BC2L-A was used for imaging experiments: E. coli and B. cenocepacia cells were incubated with the lectin, which accumulated exclusively at the surface of B. cenocepacia and within its biofilm. This study confirmed the ability of this soluble lectin to interact not only with the host mannosylated glycoproteins but also with bacterial cells participating in the biofilm matrix.
As the study of BC2L-A advanced, so did the interest in the other orthologs of lecB: bclB, bclC and bclD. Indeed, these putative proteins were longer than BC2L-A, featuring N-terminal domains with no relation to LecB. Thanks to their identification as soluble lectins, their role was considered in B. cenocepacia’s virulence studies. One study evaluated the evolution of genomic expression and chronic infection on a single patient who suffered from cepacia syndrome. (Mira et al., 2011) The transcriptomic analysis surveyed which genes were up-or down-regulated in 3 years of chronic infection. Among others, genes coding for BC2L-B and -C were up-regulated, whereas the corresponding gene for BC2L-A was down-regulated. This difference of outcomes pointed towards the possibility of secondary roles for the N-termini of these lectins.
Other studies detailed the genes regulated by quorum sensing in B. cenocepacia.(Inhulsen et al., 2012; Schmid et al., 2012) The influence of the lectins -A, -B and -C on biofilm formation was assessed in these. After proving that the operon bclACB coding for the three lectins was regulated by quorum sensing, it was also uncovered that it plays a role in maintaining biofilm structure. Indeed, gene knockout strategies confirmed that biofilm was slower to grow when the lectins were absent and structurally flawed compared to wild-type biofilm: it presented cavities and alterations in thickness and biomass. More importantly, lectin-specific knockouts revealed that all three lectins were necessary, and the lack of any one of them led to defective biofilm. This discovery hints at specific roles of each lectin, again pointing to the role of the N-termini of BC2L-B and -C. Compared to P. aeruginosa, blocking lectin action on B. cenocepacia produced a mitigated effect (malfunction instead of disruption). Nevertheless, this information remains encouraging if these lectins become targets for AAT.
Considering the growing body of data, it was clear that the discovery of LecB-like proteins featuring additional N-terminal domains was not trivial. The study of these domains could uncover information related to the role of the BC2L family in virulence and the adhesion mechanisms of B. cenocepacia. It was so that the lectin BC2L-C and its N-terminal came under close scrutiny. (Sulak et al., 2010) Their study would reveal a superlectin. (Sulak et al., 2011)
