Anatomic and chemical-physiological barriers
The skin is composed of two layers, epidermis and dermis. The epidermis consists of several layers of tightly packed cells, where the outer layer contains dead cells and waterproof protein keratin, and most of the pathogens are not capable to cross such a barrier. The underlying dermis contains sebaceous glands that produce oily secretion called sebum consisting of lactic acid and fatty acids providing the acidic pH, which inhibits the growth of many pathogens. The structure and composition of the skin makes it a formidable physical barrier, whereas mucosa does not possess as strong physical features, but it has other properties that compensate the lack of rigidity. Mucosa is composed of tight epithelial and the underlying connective tissue layers, and lines the conjunctiva, the gastrointestinal, respiratory, and urogenital tracts. These linings possess numerous non-specific defense mechanisms such as mucous secretions (tears, saliva) that wash away the microorganisms, or viscous mucus, which entraps the potential pathogens, and specialized organelles of lung epithelial cells, i.e. cilia, that expel the pathogens by its movement.
The skin and mucosal surfaces are also protected by antimicrobial substances secreted by keratinocytes or specialized epithelial cells. These substances include mucins (the glycoproteins that prevent attachment and entry of the microbes), antimicrobial enzymes and peptides. Lysozyme and phospholipase A2 are antimicrobial enzymes secreted in mucosa and responsible for the destruction of microbial cell walls and membranes, respectively. Antimicrobial peptides in mammals include defensins, cathelicidins and histatins. Defensins are small amphipatic peptides possessing a positively charged region separated from hydrophobic region. It is suggested that they function by binding to the pathogen membranes through their positive region due to electrostatic attraction, followed by insertion of the hydrophobic region into the membrane, which results in pore formation and pathogen destruction. Defensins are secreted by keratinocytes in epidermis, the cells of tongue, respiratory and urogenital tracts, and by the Paneth cells of the gut into the gut lumen.
Defensins are also secreted within the tissues by phagocytes, and they constitute one of the components of primary granules of the neutrophils. Cathelicidins are constitutively produced by neutrophils, macrophages, and by keratinocytes in the skin and epithelial cells in the lungs and intestine in response to infection. Their mechanism of action is similar to that of defensins. Histatins are produced by the parotid, sublingual, and submandibular glands in the oral cavity. They are short histidine-rich cationic peptides that are active against pathogenic fungi such as Cryptococcus neoformans and Candida albicans (Murphy, K, Geha, R, & Notarangelo, L. (2011).
The cells of innate immunity
Apart from non-specific or broadly specific counter-action of physiological component of the innate immunity, a more specific response is carried out by the innate immunity cells: macrophages, neutrophils, basophils, eosinophils, mast cells, natural killer cells (NKs), and dendritic cells (DCs). Moreover, the tissue intrusion by the microbes triggers inflammatory responses, which recruit more effector cells and molecules from the bloodstream to the infection site and serve to destroy, dilute or wall off both the injurious agents and the injured tissue [Pathak, S & Palan, U. (2005)]. All cells of immune system, together with other blood cells, are believed to develop from a common precursor in the bone marrow, a Hematopoietic Stem Cell (HSC), under the stimulation by certain cytokines (fig. 3). The differentiation of the generated progenitor cells may be either completed in the bone marrow, or the cells may mature in several stages after they leave bone marrow (DeFranco, A, Locksley, R, & Robertson, M. 2007)
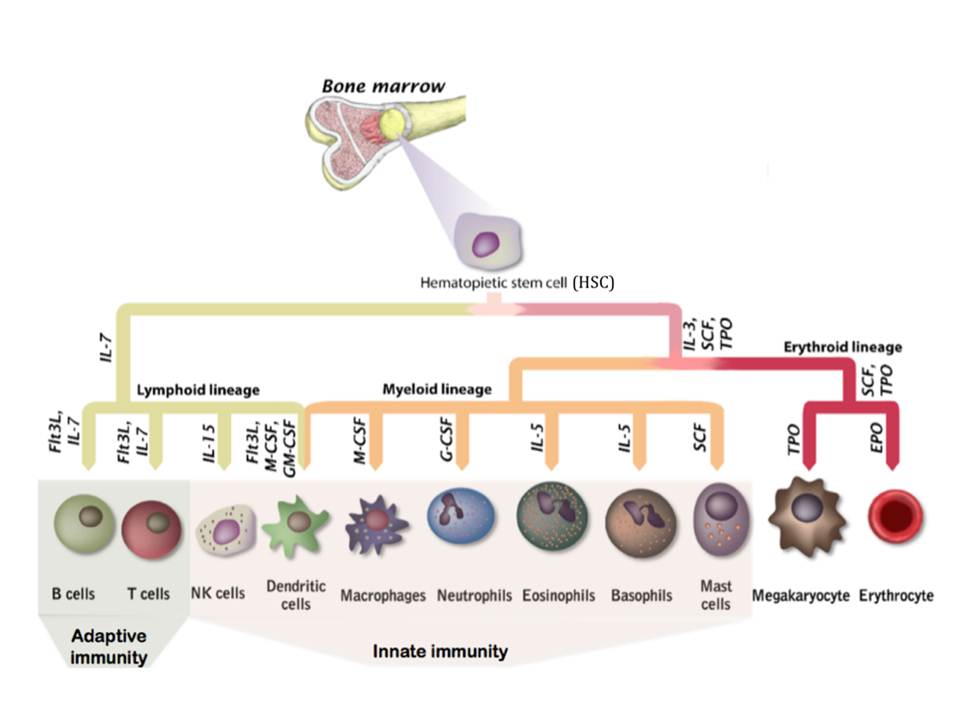
Basophils, mast cells and eosinophils have a special role in the protection of epithelial surfaces, especially the mucosa of the gastrointestinal, respiratory and urogenital tracts, against the multicellular parasites, such as helminthes. They all are characterized by cytoplasmic granules containing inflammatory and cytotoxic mediators, thus they are referred to as granulocytes. Mast cells have a sentinel role and reside in mucosal and connective tissues, while basophils and eosinophils are circulating cells recruited from the bloodstream. They can recognize microorganisms either directly or through activation by complement or lymphocyte products. These granulocytes operate by releasing the contents of their granules to the exterior upon activation, thereby either creating an environment hostile to invading organisms or directly killing the parasites (in the case of eosinophils). Moreover, basophils and mast cells release molecules including histamine that are clinically important as the mediators of allergic and pathological inflammatory responses ( DeFranco, A, Locksley, R, & Robertson, M. 2007, Paul, W. E. 2008).
The phagocytic cells of innate immunity comprise neutrophils, macrophages and immature DCs (iDCs). Neutrophils are the front-line effector cells of innate immunity : once the differentiation is complete, they circulate in a bloodstream for a few hours and after entering the tissues, they can ingest infectious microorganisms and kill them by microbicidal substances stored in specialized vesicles as well as by reactive oxygen species (ROS) generated by NOX family NADPH oxidases (Bedard, K & Krause, K.-H. 2007). These vesicles appear as granules when stained, therefore neutrophils have a name of granulocytes. They are also called polymorphonuclear leukocytes because of their characteristic feature of multilobed nucleus. Neutrophils are short-lived and die after two or three days, while macrophages are long-lived innate immunity cells that carry out immune surveillance in the tissues and play an important part in tissue maintenance. They differentiate from blood circulating monocytes as they leave the bloodstream. Like neutrophils, they ingest and destroy microorganisms. Macrophages also participate as effector cells in adaptive immune responses when activated by T lymphocytes or by antibodies secreted by B cells. Both neutrophils and macrophages release inflammatory cytokines.
Macrophages and most iDCs are sentinel cells residing in tissues, and depending on the tissue where they reside, their receptors and functional properties can vary. Apart from microbial invaders, both of these cell types sample tissues for normal tissue debris, as they possess a variety of scavenger receptors, specific for molecules characteristic to cells that have undergone apoptosis.
DCs form a link between innate and adaptive immunity, as their principal function is to induce the adaptive immunity. They may develop from both lymphoid and myeloid lineages, and a myeloid progenitor of DCs and macrophages is known as a monocyte. For a period of days to weeks immature DCs survey the peripheral tissues and operate as phagocytes to internalize the foreign microorganisms. This leads to differentiation into mature DCs (mDCs) that have the antigens presented on their surface and are no longer phagocytic. They leave the peripheral tissues to migrate into the lymphoid organs where they will encounter circulating naïve T cells and activate them. mDCs are characterized by the long branches, for which they are named : these enable to make contacts with several T cells simultaneously (DeFranco, A, Locksley, R, & Robertson, M. 2007).
