Dendritic cells comprise a diverse group of professional antigen presenting cells (APCs), which share a particular morphology, i.e. long surface membrane extensions called dendrites (fig. 8), and have a common feature of being potent stimulators of T cells to induce the adaptive immunity. The diversity of DCs is a result of a combination of several features including their origin, differentiation state, and anatomic location. The different subsets are identified by expression of surface markers, but the distinction between them is often debatable. Nevertheless, DCs can be broadly divided to myeloid (myDCs) and plasmacytoid DCs (pDCs). The latter originate from lymphoid progenitor, they resemble plasma cells, hence they are called “plasmacytoid”. myDCs are derived from monocytes and differentiated from peripheral blood mononuclear cells (PBMCs). Other DC subpopulations include Langerhan’s cells (LCs) residing in epidermis of the skin and interstitial DCs (iDCs) found in dermis of the skin and stromal compartments of mucosal tissues. While myDCs are very potent at pathogen uptake and antigen presentation, the lack of CLRs and Fc receptors on pDCs together with weak macro-pinocytosis suggest different function of pDCs, which may involve self-antigen presentation and/or it is restricted to viral antigen presentation. Besides, pDCs are the major source of type I interferons (IFN) supporting their role in antiviral immunity (Peiser, L, Mukhopadhyay, S, & Gordon, S. 2002, Williams, A. 2011).
Colored scanning electron micrograph of a dendritic cell.
All DCs express PRRs on their surface including TLRs and CLRs, but each subset has its characteristic differential expression of PRRs, therefore distinct subsets will be reactive to a certain group of PAMPs present in particular pathogens (examples in tables 1 and 2). The diversity of these receptors on DCs enables them to uptake multiple signals from their surroundings and finally to transfer them for induction of appropriate adaptive immune responses ( Peiser, L, Mukhopadhyay, S, & Gordon, S. 2002, Williams, A. 2011).
The immature skin and mucosal DCs form a dense network of resident cells, which sample their environment for foreign microorganisms. Upon pathogen recognition or in response to cytokines such as TNF-α, IL-4, GM-CSF, the maturation of DCs is triggered. This involves down-regulation of endocytic receptors, up-regulation of maturation markers (e.g. costimulatory molecules such as CD40, CD80 and CD86), raise in the levels of major histocompatibility complex (MHC) class II expression, loss of adhesiveness and induction of DC migration to lymphoid organs to present the processed antigens for T cell activation (fig. 9). Receptor-triggered DC responses also include expression of pro-inflammatory (TNF-α, IL-1, IL-6) or anti-inflammatory (IL-10) cytokines and differential production of chemokines, which results in recruitment of different T-cell subsets at the site of infection ( Lombardi, G & Riffo-Vasquez, Y. 2008).
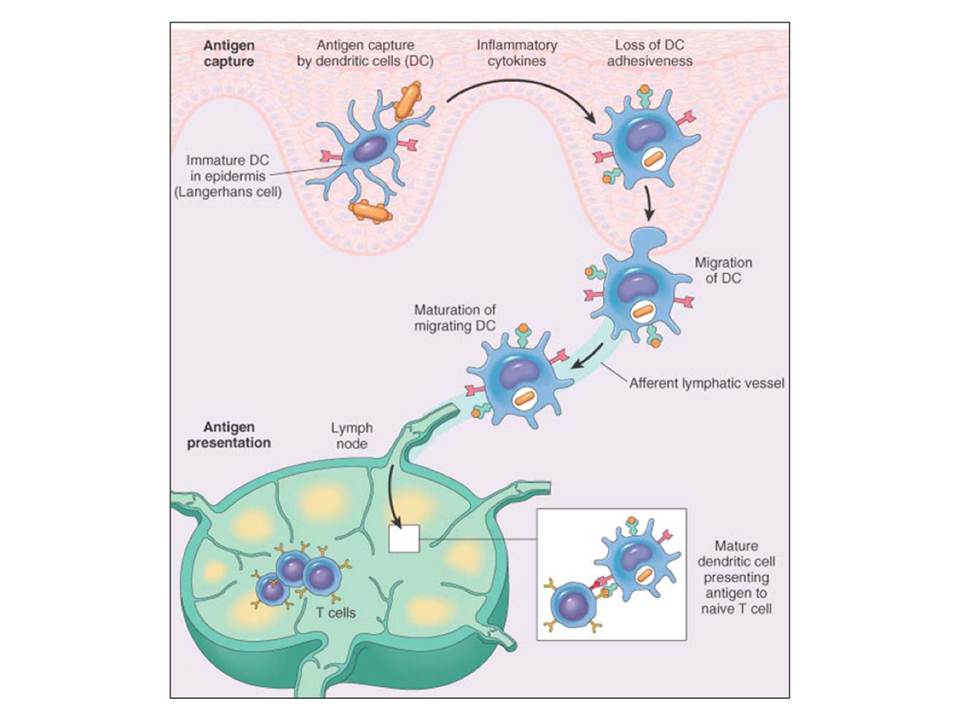
Antigen uptake, maturation and migration of dendritic cells to activate T cells.
Depending on which PRRs are triggered by pathogens, different signaling pathways are initiated in DCs. Generally, DC triggering through TLRs leads to DC activation, while PAMP recognition by CLRs result in pathogen uptake, processing, antigen presentation on MHC class I or II molecules, but also may involve modulation of DC activation (Lombardi, G & Riffo-Vasquez, Y. 2008).
On the other hand, the maturation of myDCs may be inhibited by immunosuppressive cytokines such as IL-10 and TGF-b, also by corticosteroids, cyclosporine A, and 1α,25-dihydroxyvitamin D. Such DCs may induce regulatory T cells, which maintain tolerance to self-antigens. Thus DCs have a dual function: they either boost the immune system, or dampen it leading to tolerance and maintenance of the immune homeostasis (Gessani, S & Belardelli, F. 2007).
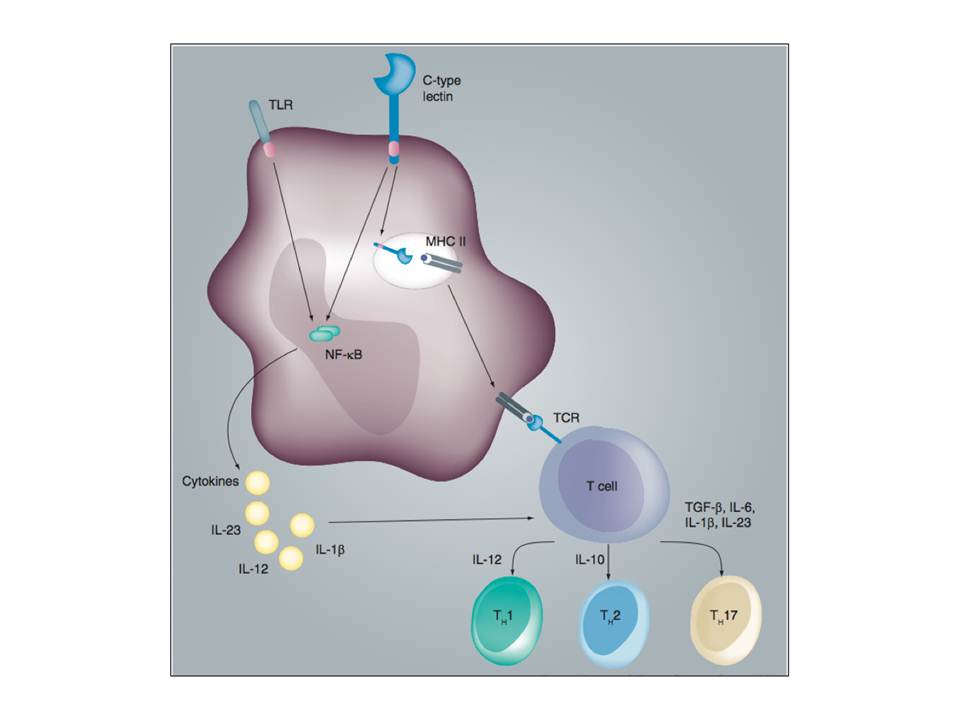
The cross-talk between TLR and CLR signaling shape the adaptive immunity.
The differentially activated DCs induce naïve CD4+ T cell differentiation into distinct T helper cells: Th1, Th2 or Th17 (fig. 10). These T helper cells are responsible for fighting different types of pathogens. Th1 produce interferon-g (IFN-γ), which activates macrophages and cytotoxic T cells to fight intracellular pathogens. Th2 cells secrete interleukin-4 (IL-4), IL-5, and IL-13, which are responsible for the activation of B cells and humoral immune responses against extracellular pathogens. Antifungal and antibacterial immunity is contributed by Th17 cells secreting IL-17, which mobilizes phagocytes (van den Berg, L. M, Gringhuis, S. I, & Geijtenbeek, T. B. H. 2012).
The information about invading pathogens or abnormal self is transferred from DCs to naïve T lymphocytes through immunological synapse – a specialized cell–cell adhesive junction characterized by stability and directed secretion. The formation of immunological synapse is initiated by adhesive contacts between the two cells that are mediated by a number of adhesion receptors (fig. 11 A, B), which facilitate to form T cell receptor (TCR) and antigen-bound MHC class I or II complexes for antigen scanning by T cells and signaling to induce appropriate adaptive immune responses (Dustin, M. L, Tseng, S.-Y, Varma, R, & Campi, G. 2006).
