The biosynthesis of glycoprotein based blood group A, B and H antigens relating to terminal glycosylation is similar to glycolipids in most aspects. However, biosynthetically blood group protein glycosylation starts very differently from glycolipids and usually involves two different pathways. The first starts with the linkage of a GalNAc residue to oxygen of a serine or threonine creating an O glycosidic bond (O-linked glycan) while the alternative second pathway involves the linkage of GlcNAc to the nitrogen of an asparagine (which is present in a specific Asn-X-Ser/Thr peptide sequence) creating an N glycosidic bond (N-linked glycan). O-linked glycans exist in several different inner cores while N-linked glycans have the same glycan precursor. The N-linked glycan may furthermore be processed into three different types : high mannose type, hybrid type, and complex type, respectivelyPaulson, 1989. These inner core structures may be terminated with peripheral core structures that define the nature of the ABO antigen. Unlike glycolipids and O-linked glycoproteins, which appear to be built sequentially from the primary saccharide, the N-linked glycoprotein first involves the transfer of a sequence of saccharides, which after trimming is able to proceed with further elongation by other glycosyltransferases Helenius, 1994Schachter, 2000. For extensive reviews on glycoprotein biosynthesis, the reader is referred elsewhere. Although both O and N-linked glycans are present on the red cell surface N–linked blood group glycans appear to be the most abundant.
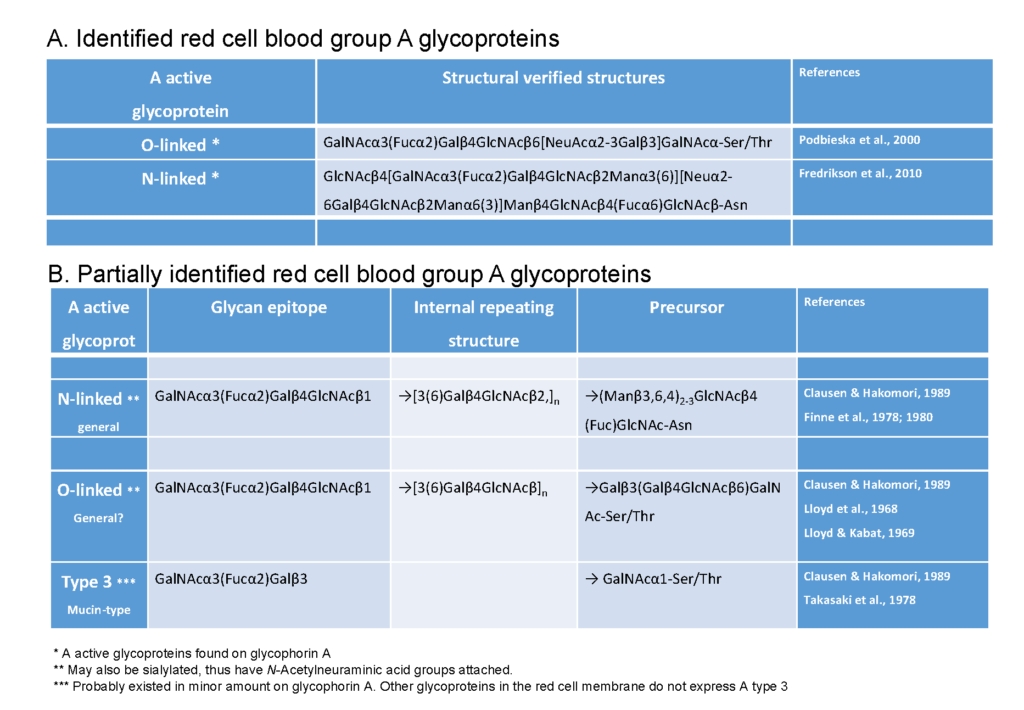
A further important distinction between glycoproteins and glycolipids is the former is immobile in the membrane, while the latter is able to move freely about in the membrane. The impact and consequences of this mobility difference are not known, but it has the potential to impact on biological interactions with antibodies and micro-organisms. Despite protein glycosylation being dominant on red cells, the actual structural identities of blood group glycoproteins isolated from red cells is very poorly resolved. Extensive analysis of the literature on blood group A glycoproteins, conclusively identified in the red cell membrane, only a few structurally identified examples were found (Table 2). Most of the blood group A glycoproteins structurally resolved have been described in other tissues/secretions Morgan & Watkins, 2000, and whether these exist on the red cell membrane is not known. Recently the existent of ABO blood group antigens in human glycophorin-A on red cells been reported Podbielska & Krotkiewski, 200. However, clearly, there is a major lack of understanding blood group A glycosylation of the red cell membrane.