Anti-ABO antibodies
A and -B antibodies are present in individuals lacking the specific ABO antigen.
The principles described in this section of antibodies against blood group A antigen are in most cases also applicable to blood group B antigen.
Polyclonal antibodies (PAb) associated with blood group A
Anti-B appears always to be associated with all healthy individuals with blood group A including those with weak A subgroups and phenotypes. Anti-A, at least anti-A that is directed against the A type 2 antigen is also usually absent, although anti-A-like antibodies that will react with trisaccharide A antigen are frequently present Obukhova et al., 2012. In contrast anti-A1, an antibody that reacts with A1 cells and not A2 cells is frequently associated with subgroups and weak phenotypes of A. The actual specificity of anti-A1 is uncertain but on the basis of what is known about the A1 and A2 phenotypes it is probable that anti-A1 specificity is directed against A type 4 (globo-A) and not the closely related A type 3 antigen (figure 4). This is supported by indirect evidence that the A type 3 antigen is observed in weak subgroups of A and that A type 4 antigen is usually not observed unless the weak subgroup has an A1 allele Svensson et al., 2011Svensson et al., 2009. However, it is equally possible that anti-A1 is directed against some as yet undefined glycans.
A antigen detecting serologic reagents
The detection of A antigens on red cells is usually as a consequence of reactivity of red cells with either antibodies or lectins. Antibodies can be loosely divided into two types ; polyclonal antibodies (PAb) obtained from serum (either as whole serum or affinity purified antibodies) and monoclonal antibodies (MAb) (either as single clones or formulated blends). Serological blood typing lectins, in contrast, are non-immune proteins from plants and animals that react with specific carbohydrates or carbohydrate sequences and are able to induce direct agglutination and obtain specificity through dilution.
Common to all of these reagents is a significant level of cross-reactivity with related compounds Obukhova et al., 2012Oriol et al., 1990, which in most cases is either unknown, poorly characterized and usually not appreciated.
PAbs prepared from the immunized serum of humans or animals, recognize multiple epitopes and are less standardized and concentrated than most monoclonal reagents. Recently it has been shown that the specificity scope of PAbs may be narrower than originally anticipated Obukhova et al., 2012.
Historically, weak phenotypes were more frequently detected by PAbs than with today’s modern MAbs, primarily because the latter are more concentrated. The higher the concentration of the antibody the better their ability to detect low levels of antigen. The most important PAbs for detecting weak phenotypes were those with anti-A,B specificity. These antibodies appear to recognize two sequences, specifically a terminal sequence and an internal sequence.
Most routine-used ABO MAbs are formulated as blends and the clones used are selected to be broadly reactive with all appropriate ABO antigens. Probably many of the stronger historically weak-subgroups would go undetected today and simply type as a normal phenotype. In contrast to the PAb anti-A,B reagents with an ability to detect weak phenotypes of the ABO system, monoclonal anti-AB reagents are poor at this, probably because the ability of mice to make anti-A,B is compromised by them being linear B antigen positive. To compensate for this almost all serological MAb anti-AB reagents are blends.
A few restricted specificity anti-A MAbs exist directed against antigens such as A-type 3/4 and anti-ALeb but these are not usually selected for routine serological reagents.
It should also be appreciated that we usually do not know the full activity profile of a particular MAb and that in some circumstances reactivity may be misinterpreted due to unknown reactivity Svensson et al., 2013.
Phenotypes of blood group A
The principles described here for blood group A antigen are in most cases applicable to blood group B and AB phenotypes.
A phenotype is the composite of observable characteristics. The primary mechanism for observing and describing phenotypes in red cell serology is red cell agglutination characteristics with a defined antibody. Therefore any consistent observable pattern in the reactivity of anti-A reacting with A antigen on red cells, is potentially an A phenotype, regardless of what genetic or environmental factors were or were not involved. The primary factor relating to these phenotypes is a quantitative variation in A antigen expression on the red cells although there is strong evidence for qualitative differences as well Svensson et al., 2005.
The “strong” phenotypes A1 and A2
The A1 and A2 phenotypes representing >99% of all A blood group phenotypes strongly express A antigen and are easily detected by all valid serological methods. The index A1(A101) allele, has eight additional variant alleles, while A2 has 20 variants (see BGMUT database). The blood group A1 phenotype has the highest expression of A antigens on red cells (about 1 million antigens) and is thus considered the index against which all the other variants are compared. The A2 phenotype has about a quarter of the A antigens present on A1 cells. It should be noted that antigen numbers reported in the literature are potentially on the low side (unpublished observations).
The mechanism resulting in the A2 phenotype is a genetically defined enzyme variation resulting in an elongated and less efficient glycosyltransferase that requires different pH optimum, Km values and ions to transfer the N-acetylgalactosamine saccharide to the acceptor molecule.
The chemical basis, for the A1 and A2 phenotypes, has been debated for many years. However more recently it was concluded that in addition to the primary quantitative differences, qualitative differences also are present. Blood group A type 3 glycolipids are similarly expressed in A1 and A2, but in lesser amount in the latter. However, the A-7-4 glycolipid present in the A1 phenotype is undetectable or expressed in very small amount in A2 Svensson et al., 2009. On this basis, it should be anticipated that anti-A1 has the specificity of anti-A type 4. Furthermore, the contribution of the glycoproteins to phenotypic differences is unknown.
The Aint phenotype
An intermediate phenotype has been claimed to exist in the realm between the A1 and A2 phenotypes. This phenotype, called Aint is represented by weak reactions with anti-A1 and reactivity with anti-H and has been associated with a variant glycosyltransferase activity Yoshida et al., 1982.
The “weak” ABO subgroups/phenotypes
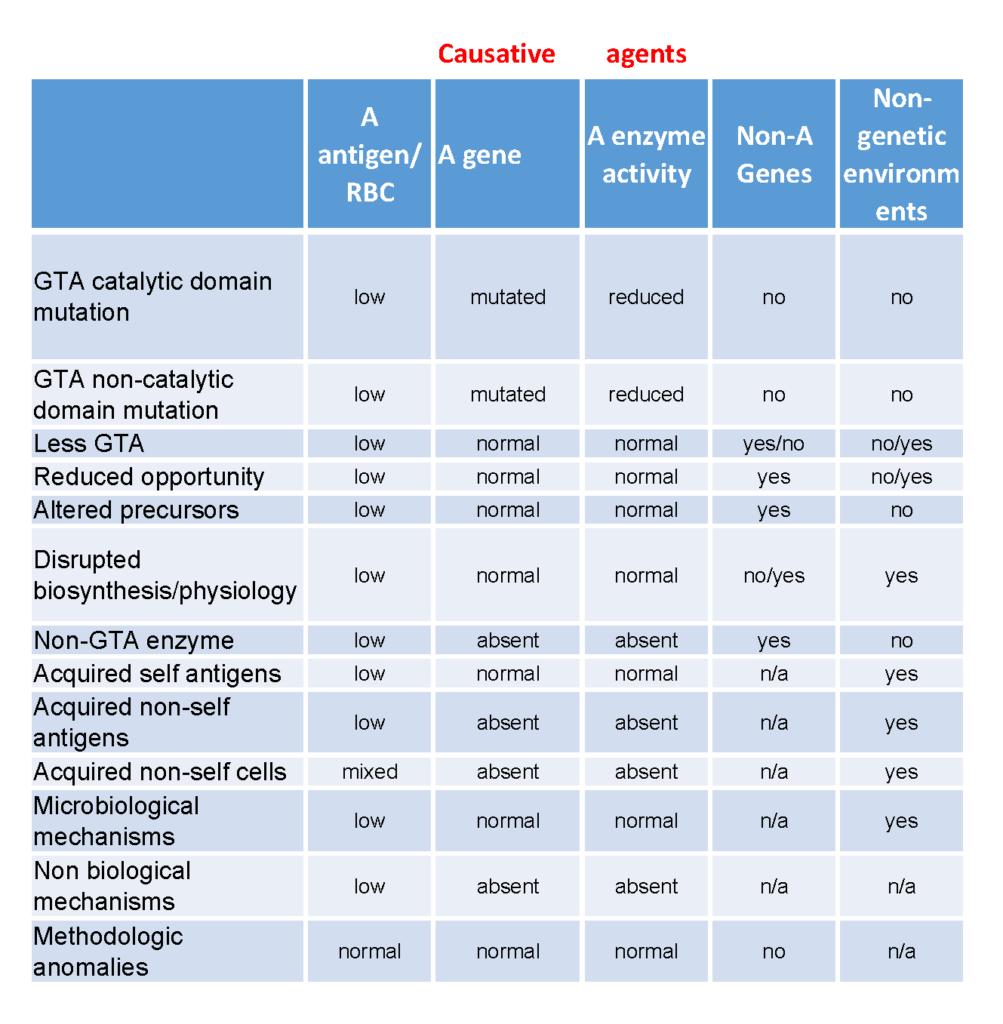
The term “weak” refers to the relatively weak strength of a serological reaction observed when compared with the two dominant “strong” phenotypes (e.g. A1 or A2). However, differentiating the weak ABO phenotypes and defining the term “subgroup” is complex and ambiguous. In many instances, the ABO antigen level is defined by the individuals ABO genotype, but reduced antigen levels can be alternatively determined by non-ABO genetic and/or environmental factors or sensitivity and specificity of the detection system. In order to rationalize the different mechanisms for obtaining weak phenotypes, we have organized them into categories based on their genetic and environmental backgrounds (Table 3). The premise of this informal classification is to define a “weak subgroup” as a phenotype that has to have arisen from reduced blood group A glycosyltransferase activity background while those from unknown or other backgrounds are simply termed as “weak phenotypes”. Phenotypes (where the red cells react with anti-A) arising from backgrounds where there is no A antigen on the red cells are termed “false phenotypes”.
From a simplistic perspective, there is essentially only one way to have a weak subgroup phenotype, and that is to have reduced levels of detectable blood group A antigen on red blood cells. However, there are several mechanisms which could result in phenotypes representing or resembling decreased expression of A antigen on the red cell. These include :
1. Blood group A glycosyltransferase (which may also have altered antigen synthesis characteristics) with abnormal catalytic domain
2. Blood group A glycosyltransferase (which may also have altered antigen synthesis characteristics) with normal catalytic domain but impaired function (like A2)
3. Less of a normal glycosyltransferase
4. Normal glycosyltransferase but reduced opportunity to make antigen – e.g. reduced available precursors, increased Golgi turnover, etc.
5. Normal glycosyltransferase but altered precursors – e.g. linear A antigens on neonatal cells.
6. Disrupted biosynthesis/physiology due to disease e.g cancer and LAD type II
7. Lack a true A glycosyltransferase, but have another glycosyltransferase that make A or A-like antigens (either within ABO or outside ABO), e.g. B(A) and FORS
8. Acquiring self antigens from the environment – e.g. para Bombay acquiring A type 1 glycolipids
9. Acquired non-self antigens from the environment – e.g. acquiring glycolipids via a plasma infusion
10. Acquired non-self cells from the environment – e.g. transfusion, transplantation and chimera
11. Microbiological mechanisms – enzymes secreted by bacteria in infections can decrease A antigen levels (and make acquired B antigen)
12. Non-biological biological mechanisms including artificial modification of cells with enzymes (adding or removing) or synthetic constructs
13. Methodologic anomalies due to reagent and system sensitivity and specificity
14. Any combination of the above
This chapter described the biosynthetic pathways and the structures of the ABO blood group antigens ; its weaker subgroups and antibody response.
The focus was also set on natural and unnatural mechanisms resulting in the biosynthesis and presence/absence of blood group antigen on red cells. The principles described here for blood group A antigen are in most cases applicable to blood group B and AB antigens.
