Glycosyltransferases are type II membrane proteins, with a short N-terminal cytoplasmic tail, a hydrophobic transmembrane region (TM) and the C-terminal catalytic domain in the lumen of the endoplasmic reticulum (ER) and Golgi. The cytoplasmic, transmembrane and stem regions of glycosyltransferases may specify their functional localization and stability within the Golgi Grabenhorst & Conradt, 1999. Reports have indicated that changes in the TM- and stem-regions may also affect the specificity of the enzymes de Vries et al., 1995.
The expression of glycosyltransferases in tissue and cells is specific and highly regulated during differentiation and proliferation. In general, the expression appears to be regulated at the transcriptional level. Soluble forms of glycosyltransferases have been demonstrated and purified from milk, serum and other body fluids and increased serum levels have been noted in different diseases and in inflammation Kleene & Berger, 1993. The origin of some of these enzymes has been shown to be a result of proteolytic cleavage between the catalytic domain and the TM-region Paulson & Colley, 1989. Taken together, alterations in other regions of the protein than the catalytic domains including post-translation modifications may impact the final glycosylation event. In addition, environmental factors such as ion homeostasis and pH also strongly influence the glycosylation machinery.
The ABO transferase
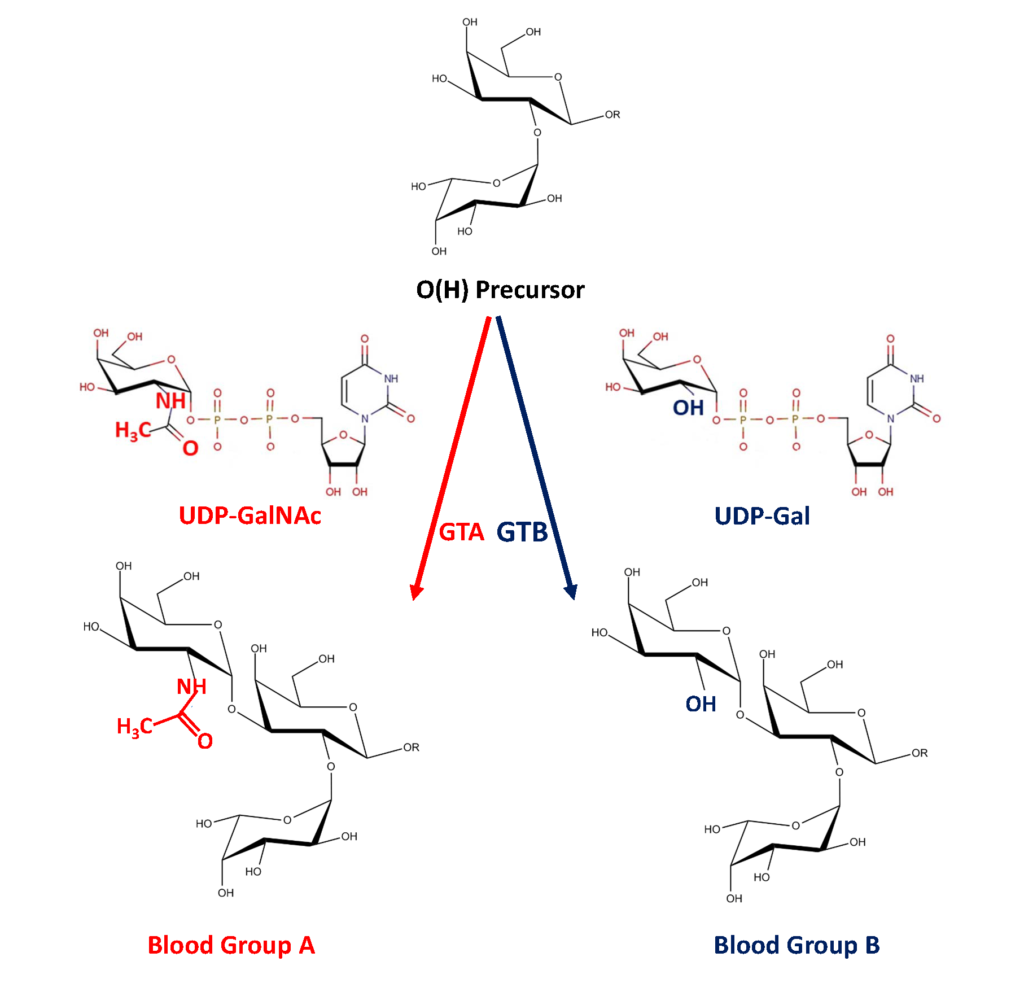
The ABO glycosyltransferases encoded by the ABO gene consists of 354 amino acids and resides in the trans-Golgi network. The soluble secreted form, proteolytically cleaved from the Golgi, found in body fluids consists of amino acids 54-354 (Figure 1). The ABO transferases transfer a UDP-N-acetyl-galactosamine (UDP-GalNAc) residue in a α1,3 linkage (α3GalNAc) to the fucosylated glycan precursor, H-antigen, forming the A-antigen, and is therefore denoted the A-transferase (GTA : EC.2.4.1.40) (Figure 2).
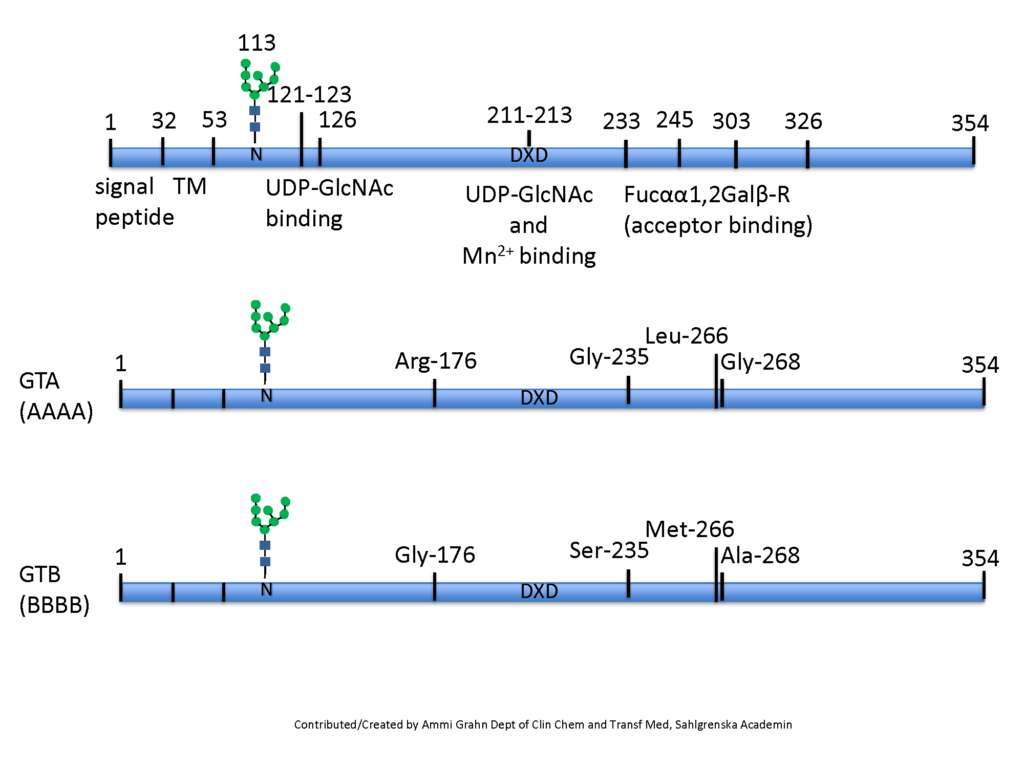
Polymorphism in the ABO gene results in ABO transferases with different substrate specificities (described below). Alteration of the amino acid 176 Arg>Gly, 235 Gly>Ser, 266 Leu>Met and 268 Gly>Ala changes the enzyme donor specificity towards UDP-galactose (UDP-Gal) but not the linkage or acceptor specificity (Figure 1). This results in the formation of the B-antigen and the enzyme is therefore denoted the B-transferase (GTB : EC.2.4.1.37) (Figure 2). The blood group O is caused by a deletion of a nucleotide near the N-terminus of the protein, which results in a frame-shift and truncated inactive enzyme or any other mutation which affects the catalytic function of the enzyme. Thus, the structure of the blood group O is an unmodified H-antigen. The term ABH for ABO blood groups antigens is more appropriate especially on other tissue than erythrocytes.
As the unmodified ABO transferase exhibits α1,3GalNAc transferase activity it belongs to the glycosyltransferase family 6 (GT6). Most glycosyltransferases harbor a conserved DXD motif involved in cation binding (Figure 1). In the GTA and GTB, the manganese ion (Mn2+) interacts with the beta-phosphate group of the UDP moiety of the donor monosaccharide and may have a role in catalysis. The GTA and GTB are N-glycosylated and mutations within these sites may induce degradation of the enzymes or prevent secretion. Both GTA and GTB form stable homodimers in aqueous solution at neutral pH Soya et al., 2009Nagai, et al., 1978Nagai et al., 1978.
Interactive display of the 3D structure of GTA (PDB 1LZI)
Mouse on ? will guide you to rotate, zoom, move,.. the molecule.
The dimerization is shown to occur via noncovalent intermolecular interactions. Thus, mutations within these sites may influence the dimerization, stability, and activity of the enzymes. Both GTA and GTB show a similar affinity for the acceptor substrate Fucα2Galβ-R in the presence of UDP in the donor site. Leu/Met 266 and Gly/Ala 268 define the specificities of the GTA and GTB enzyme activities. For GTA the Leu 266 interacts with the acetamido group of UDP-GalNAc donor and a Met at amino acid 266 in GTB interacts with the hydroxyl group of the UDP-Gal Patenaude et al., 2002. GTA shows an affinity towards both UDP-GalNAc and UDP-Gal (0.4%) whereas GTB only shows an affinity towards UDP-Gal Patenaude et al., 2002. Combinations of polymorphism within these sites such as Arg-176 (A), Gly-235 (A), Leu-266 (A) and Ala-268 (B), ABBA, AABB, BBBA and BABA may occur which results in cis-AB hybrid enzymes with dual donor specificity. The BAAA, BBAA and ABAA are preferentially forming the A antigen whereas the AABA, ABBA, BBBA and BABA form both the A and B antigens (Figure 1 and 2). The BBBB (GTB) is the only variant that preferentially forms the B antigen, although it is known to be able to slowly synthesize A antigens, which also has been identified on normal blood group B erythrocytes Goldstein et al., 1989Palcic et al., 2001. In the blood group A2, the enzyme is less effective, due to amino acid change 156 Pro>Leu, resulting in less synthesized A-antigens. Unfortunately, very little has been studied regarding the enzyme activity due to different SNPs and the resulting terminal glycan. Changes of single amino acids may not only influence substrate specificity of the enzyme but may also interfere with the enzyme activity or acceptor specificity such as transfer of a GalNAc residues to other structures than the H-antigen resulting in unnatural structures. So far the limited enzymatic studies have been performed on soluble GTA and GTB recovered from plasma or recombinantly expressed in E. coli systems and the enzymatic properties of the membrane-bound GTA and GTB has not been characterized. Moreover, the significance of the soluble GTA and GTB is an open question.
