Heparan sulfates are complex polysaccharides that mediate the interaction with a broad range of protein ligands at the cell surface. A key step in heparan sulfate biosynthesis is catalyzed by the bi-functional glycosyltransferases EXT1 and EXT2, which generate the glycan backbone consisting of repeating N-acetylglucosamine and glucuronic acid units. The molecular mechanism of heparan sulfate chain polymerization remains, however, unknown. The article reports the results of a cryo-electron microscopy structure of human EXT1-EXT2. (PDB accession code 7ZAY) They reveal the formation of a tightly packed hetero-dimeric complex harboring four glycosyltransferase domains. A combination of in vitro and in cellulo mutational studies reveals the functional role of the four catalytic sites.
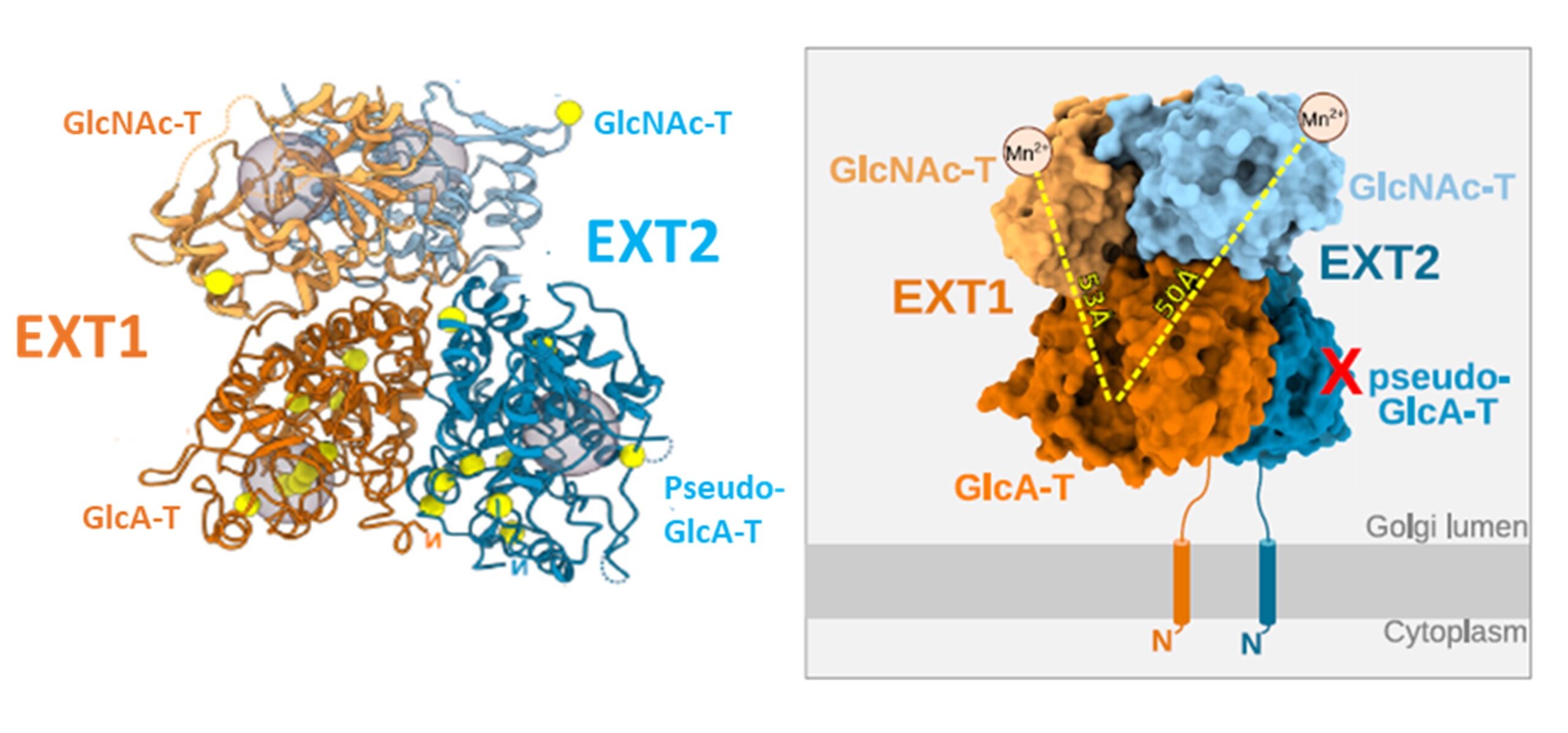
While EXT1 can catalyze both glycosyltransferase reactions, the results indicate that EXT2 might only have N-acetylglucosamine transferase activity. These findings provide mechanistic insight into heparan sulfate chain elongation as a non-processive process. They lay the foundation for future studies on EXT1-EXT2 function in health and disease.