The emergence of the betacoronavirus, SARS-CoV-2 that causes COVID-19, represents a significant threat to global human health. Vaccine development is focused on the principal target of the humoral immune response, the spike (S) glycoprotein, that mediates cell entry and membrane fusion. SARS-CoV-2 S gene encodes 22 N-linked glycan sequons per protomer, which likely play a role in immune evasion and occluding immunogenic protein epitopes. Here, using a site-specific mass spectrometric approach, the authors reveal the glycan structures on a recombinant SARS-CoV-2 S immunogen. This analysis enables mapping of the glycan-processing states across the trimeric viral spike.
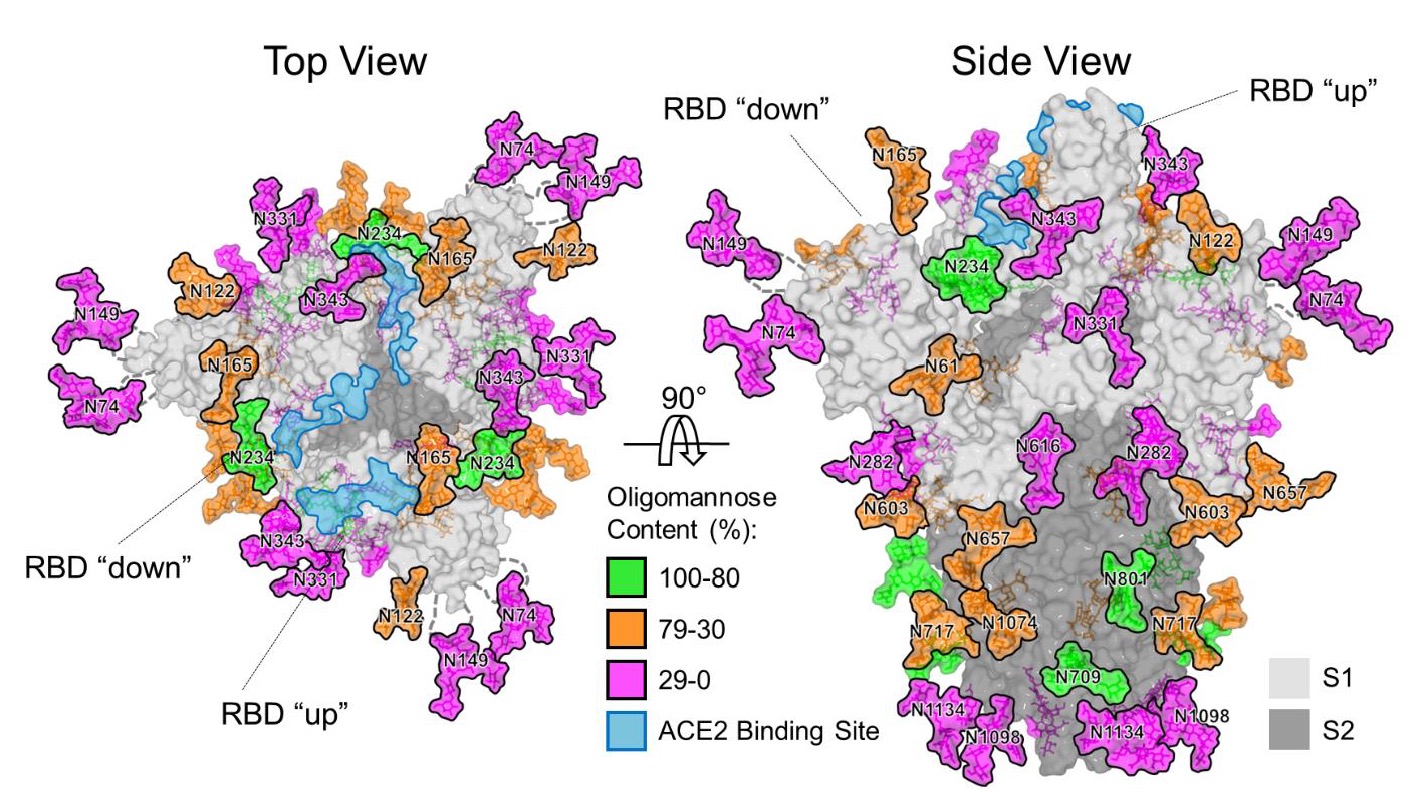
The authors show how SARS-CoV-2 S glycans differ from typical host glycan processing, which may have implications in viral pathobiology and vaccine design.




