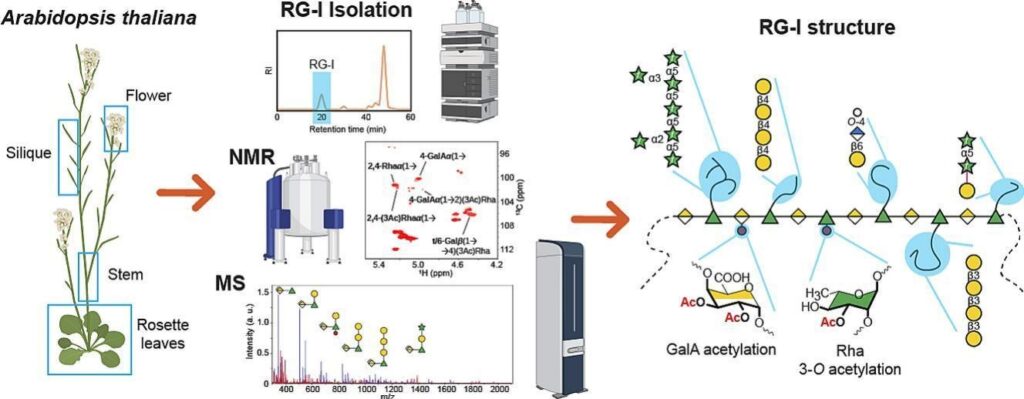
Pectin can be divided into four distinct structural categories: homogalacturonan, xylogalacturonan, rhamnogalacturonan I (RG-I), and rhamnogalacturonan II. While the structural diversity of homogalacturonan, xylogalacturonan, and rhamnogalacturonan II is well understood, the structural features of RG-I remain unclear. In this study, we used a variety of analytical techniques to conduct a detailed structural analysis of RG-I in the model species Arabidopsis thaliana. Starting with highly purified RG-I extracted from various Arabidopsis tissues, we used comparative linkage analysis, nuclear magnetic resonance spectroscopy, and mass spectrometry to analyze enzymatically digested RG-I oligosaccharides. In addition to the presence of the canonical α-1,5-arabinan, β-1,4-galactan, β-1,6-galactan, and arabinogalactan RG-I side chains of varying lengths, our results demonstrate that a significant proportion of the β-1,6-galactan is terminated by either 4-O-methyl β-glucuronic acid (GlcA) residues or, to a lesser extent, by β-GlcA lacking the Me-ether group. Notably, O-acetylation of RG-I GalA residues is a minor modification, with only 10% of the backbone Rha residues being 3-O-acetylated. Furthermore, most of the acetylated Rha residues are additionally branched with β-galactose substituents. Taken together, the combined results of these different analytical techniques represent the most comprehensive structural overview of Arabidopsis thaliana RG-I to date.