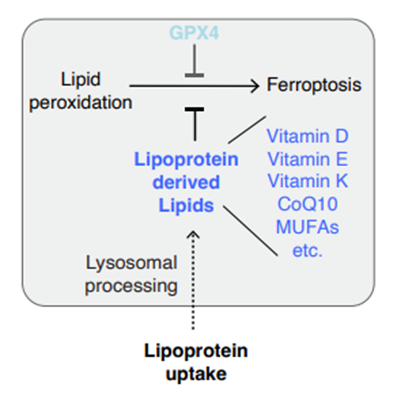
Lipids are essential components of cancer cells due to their structural and signalling roles. To meet metabolic demands, many cancers take up extracellular lipids; however, how these lipids contribute to cancer growth and progression remains poorly understood. Using functional genetic screens, the authors identify uptake of lipoproteins—the primary mechanism for lipid transport in circulation—as a key determinant of ferroptosis sensitivity in cancer. Lipoprotein supplementation robustly inhibits ferroptosis across diverse cancer types, primarily through the delivery of α-tocopherol (α-toc), the most abundant form of vitamin E in human lipoproteins. Mechanistically, cancer cells take up lipoproteins through a pathway dependent on sulfated glycosaminoglycans (GAGs) linked to cell-surface proteoglycans. Disrupting GAG biosynthesis or acutely degrading surface GAGs reduces lipoprotein uptake, sensitizes cancer cells to ferroptosis, and impairs tumour growth in mice. Notably, human clear cell renal cell carcinomas—a lipid-rich malignancy—exhibit elevated levels of chondroitin sulfate and increased lipoprotein-derived α-toc compared with normal kidney tissue. Together, the study establishes lipoprotein uptake as a critical anti-ferroptotic mechanism in cancer and implicates GAG biosynthesis as a therapeutic target.