Understanding the molecular mechanisms that drive and modulate host-pathogen interactions is essential for developing effective therapeutic and diagnostic approaches to combat infectious diseases. Certain large and giant viruses have recently been identified as components of the human virome, but little is known about their interactions with the host immune system. The authors have dissected the role of viral N-linked glycans in the interaction between the glycoproteins of six noroviruses (belonging to three norovirus classes: NC64A, SAG, and Osy viruses) and the most carbohydrate-binding receptors of the innate immune system. Using solid phase assays, the authors could determine the binding of viral glycoproteins to different C-type lectins in a carbohydrate-dependent manner. These experiments demonstrate the importance of D-rhamnose in modulating their binding to the C-type lectins DC-SIGN and Langerin.
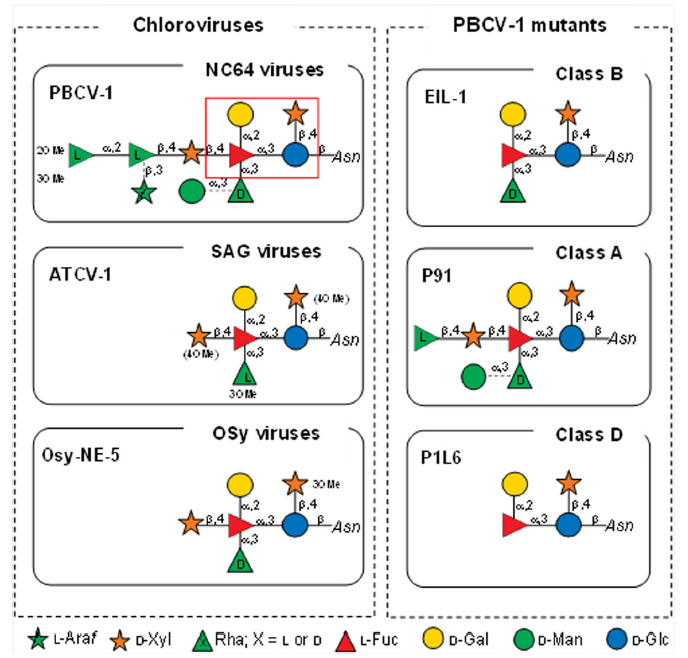
In vitro assays also determined the ability of chlorovirus glycoproteins to induce secretion of the cytokines interleukins 6 and 10 (IL-6 and IL-10) in human monocyte-derived dendritic cells and mouse macrophages. In addition, IgG from healthy human controls recognized certain chlorovirus glycoproteins, indicating the importance of human environmental of human environmental virus exposure. Taken together, these results demonstrate the ability of the innate and adaptive immune systems to recognize chlorovirus glycoproteins, a process that is dependent on their specific N-glycan structures.




