Extracellular biophysical properties have particular implications for various cellular behaviours and functions, including growth, motility, differentiation, apoptosis, gene expression, cell-matrix and cell-cell adhesion, and signaling, including mechanotransduction. Cells not only respond to distinct mechanical cues from the extracellular matrix (ECM)? They can manipulate the mechanical properties of the matrix in parallel with biological properties, thereby interfering with downstream matrix-based cues in both physiological and pathological processes. Bidirectional interactions between cells and (bio)materials in vitro can intentionally or unintentionally alter cell phenotype, mechanotransduction and ECM structure. Interactions between cell and matrix mechanics in vivo are of particular importance in a variety of diseases, including cancer, Stiffness values change between normal and cancerous tissue; even shear flow can increase from normal to cancerous tissue.
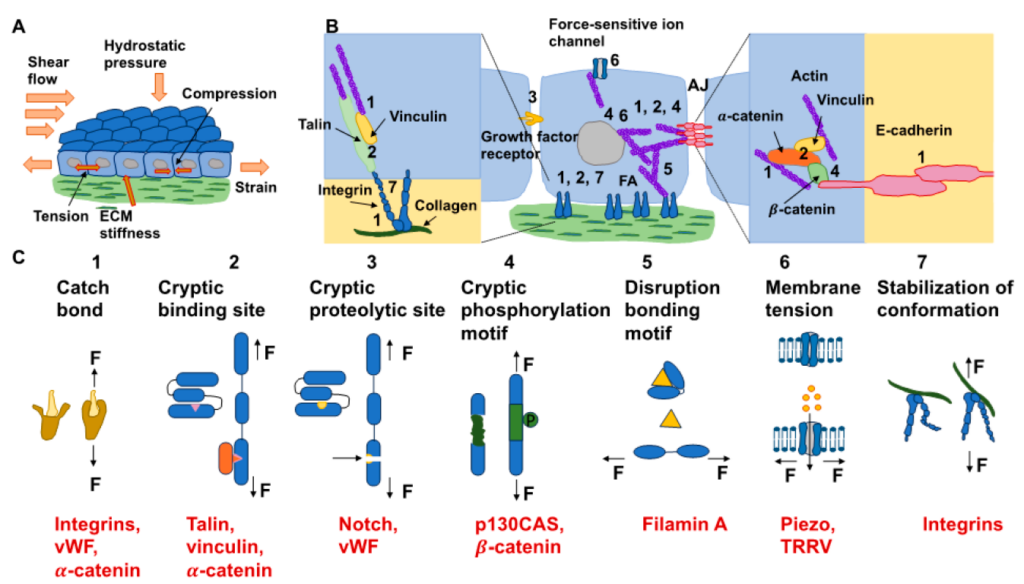
forces and can apply them to their surrounding environment, which exhibits various mechanical characteristics. (B) The kinds of mechanisms of mechanotransduction and possible ones (dotted lines) for mechanosensory proteins and aggregates of proteins within cells are sketched. FAs and AJs both comprise numerous mechanosensory proteins exhibiting specific mechanotransduction mechanisms (see insets). Even though AJs are solely sketched, it is assumed that other cell–cell adhesions, such as desmosomes and tight junctions, can transfer the forces in a similar way. The envelope of the nucleus comprises distinct proteins and protein assemblies that react to the tension of the nuclear membrane, such as the nuclear pore complex, or they are phosphorylated due to forces, such as emerin and lamin. It is still not yet clear whether these proteins are mechanosensors. Even though the gating role of force-sensitive ion channels is driven by force-based alteration in the tension of the membrane, multiple ion channels are directly controlled through forces that are transferred by the linked actin cytoskeleton of the cell. (C) Various mechanisms of mechanotransduction over specific mechanosensors, such as catch bonds (1), cryptic binding sites (2), cryptic proteolytic sites (3), cryptic phosphorylation motifs (4), disruption bonding motifs (5), membrane tension (6), and stabilization of conformation (7). The black arrows indicate the direction of the force (F) acting on the molecules or structure. Examples for each of the seven types are shown in red.
The review focuses on new areas of activity in studying tumors at a range of biological length scales. The complexity of the interactions between the ECM and cancer cells is reduced to common features of different cells. These features are highlighted to identify the main pathways of interaction. All this will contribute to standardizing mechano-transduction models and approaches, ultimately improving understanding of complex interactions. Finally, both the in vitro and in vivo effects of this mechanics-biology pairing will have important insights and implications for clinical practice.




