The SARS-CoV-2 spike glycoprotein mediates virus attachment to human host cells by binding angiotensin-converting enzyme 2 (ACE2) and heparan sulfate (HS) proteoglycans. To elucidate these interactions’ structure, dynamics, and functional consequences, the authors carried out microsecond-long all-atom molecular dynamics simulations, followed by random acceleration molecular dynamics simulations, of the fully glycosylated spike: ACE2 complex with and without heparin chains bound.
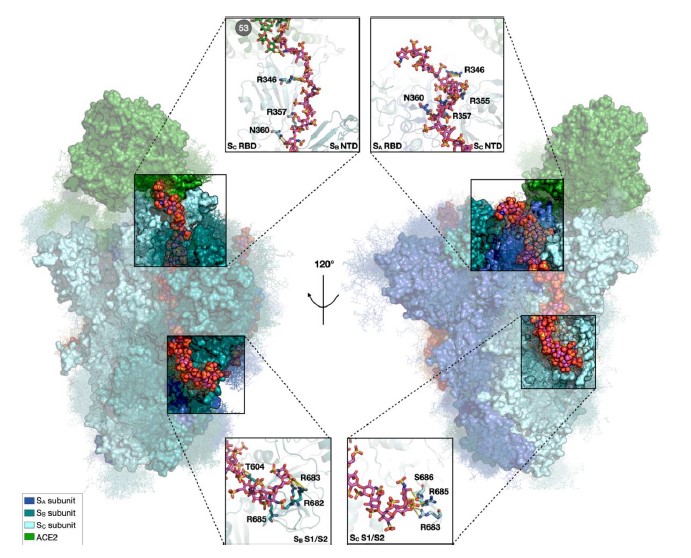
Heparin, as a model for HS, promotes structural and energetic stabilization of the active conformation of the spike receptor binding domain (RBD) and reorientation of ACE2 toward the N-terminal domain in the same spike subunit as the RBD. Spike and ACE2 N-glycans exert synergistic effects, promoting better packing, strengthening the protein-protein interaction, and prolonging the residence time of the complex. ACE2 and heparin-binding trigger rearrangement of the S2 functional site through allosteric interdomain communication. Thus, HS has a multifaceted role in facilitating SARS-CoV-2 infection.