Aberrant glycosylation, a characteristic feature of cancer, plays a pivotal role in shaping tumor behavior. Glycans, emerge as promising targets for novel clinical biomarker development, offering a specific repertoire of targets for therapeutic intervention. Various mechanisms of aberrant glycosylation culminate in the formation of tumor-associated carbohydrate antigens (TACAs), which present viable targets for selective cancer-targeting therapies. Notable TACAs include truncated O-glycans (such as Tn, TF, and sialyl-Tn antigens), gangliosides (including GD2, GD3, GM2, GM3, and fucosyl-GM1), globo-series glycans (like Globo-H, SSEA-3, and SSEA-4), Lewis antigens, and polysialic acid.
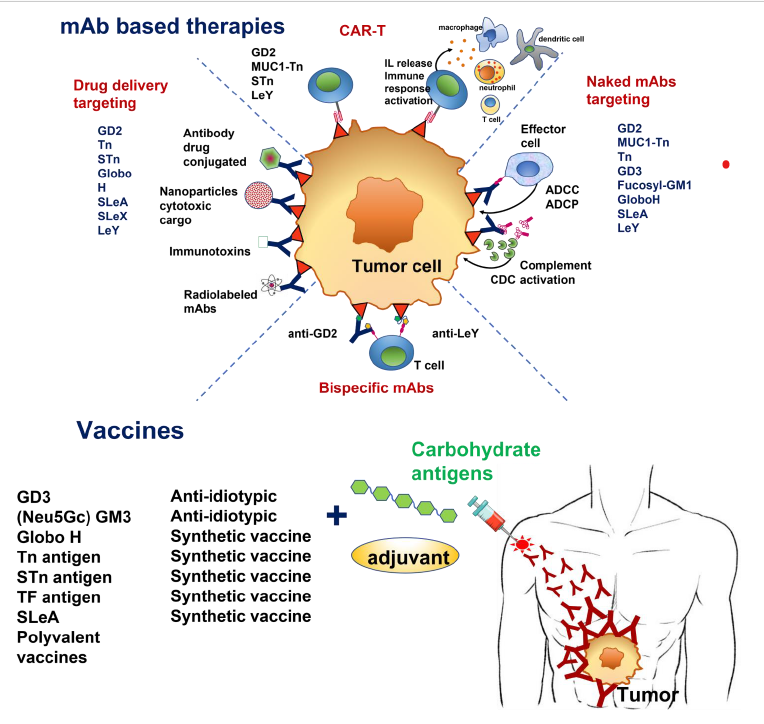
TACAs (Tumor Associated Carbohydrate Antigens). mAbs (monoclonal antibodies); CAR-T
(Chimeric antigen receptor-T); STn (sialyl Tn); SLeA (sialyl Lewis A); SLeX (sialyl Lewis X); LeY
(Lewis Y); ADCC (Antibody-Dependent Cell-mediated Cytotoxicity); ADCP (Antibody-Dependent
Cellular Phagocytosis); CDC (complement-dependent cytotoxicity).
This comprehensive review delves into the strategies employed in cancer immunotherapy targeting TACAs. It encompasses diverse approaches, ranging from the development of antibodies tailored to specific TACAs, the formulation of vaccines, to the engineering of chimeric antigen receptor (CAR) T cells. Notably, certain approaches, such as anti-GD2 antibodies, have already received approval for clinical use.
Furthermore, the review provides insights into the antitumor mechanisms underlying various TACA-targeting strategies, showcasing findings from selected clinical trials. It underscores the promising horizons that have unfolded due to recent advancements in cancer control technologies, highlighting the potential of TACA-targeted immunotherapies in reshaping the landscape of cancer treatment.




