Streptococcus gordonii is a Gram-positive bacterial species that typically colonizes the human oral cavity, but can also cause local or systemic diseases. Serine-rich repeat (SRR) glycoproteins exposed on the S. gordonii bacterial surface bind to sialylated glycans on human salivary, plasma, and platelet glycoproteins, which may contribute to oral colonization as well as endocardial infections. Despite a conserved overall domain organization of SRR adhesins, the Siglec-like binding regions (SLBRs) are highly variable, affecting the recognition of a wide range of sialoglycans. SLBR-N from the SRR glycoprotein of S. gordonii UB10712 possesses the remarkable ability to recognize complex core 2 O-glycans.
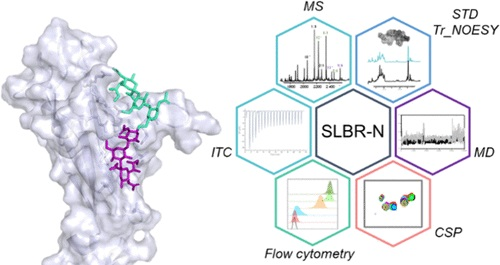
The authors employed a multidisciplinary approach to investigate the ligand specificity and binding preferences of SLBR-N when interacting with mono- and disialylated core 2 O-glycans. They determined how SLBR-N preferentially binds branched α2,3-disialylated core 2 O-glycans: a selected conformation of the 3′SLn branch is accommodated into the main binding site, driving the sTa branch to further interact with the protein. At the same time, SLBR-N assumes an open conformation of the CD loop of the glycan-binding pocket, allowing one to accommodate the entire complex core 2 O-glycan. These findings establish the basis for the generation of novel tools for the detection of specific complex O-glycan structures and pave the way for the design and development of potential therapeutics against streptococcal infections./
Siglec-Like SLBR‑N (SLBRUB10712) of Streptococcus gordonii