Glycosaminoglycans (GAGs) play a critical role in tissue homeostasis by regulating the activity and diffusion of bioactive molecules. Incorporating GAGs into biomaterials has become a widely adopted strategy in medical applications. This is due to their biocompatibility and ability to control the release of bioactive molecules. Nevertheless, immobilised GAGs on biomaterials can elicit different cellular responses compared to their soluble forms, highlighting the need to understand the interactions between GAGs and bioactive molecules within engineered functional biomaterials. The control of critical parameters such as GAG type, density and sulphation allows GAG functions to be precisely defined within the context of the biomaterial. Specific tissue properties can be better mimicked, allowing the tailor-made design of GAG-based biomaterials for specific medical applications.
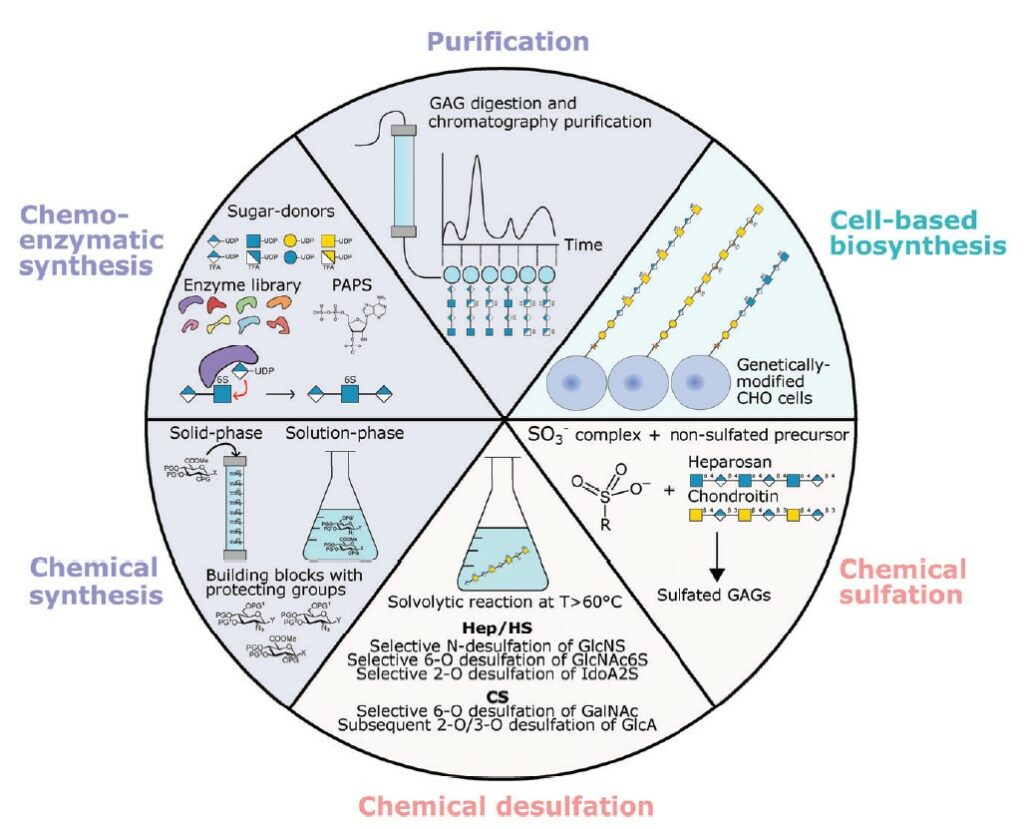
However, this requires access to pure and well-characterised GAG compounds, which remains challenging. This review article focuses on the different strategies used in producing defined GAGs and the high-throughput approaches used to study GAG-growth factors interactions and the quantification of cellular responses on GAG biomaterials. These automated methods hold great promise for improving the understanding of the diverse functions of GAGs. In the future, the scientific community is encouraged to adopt a rational approach to designing GAG-based biomaterials, considering the in vivo properties of the target tissue for medical applications.




