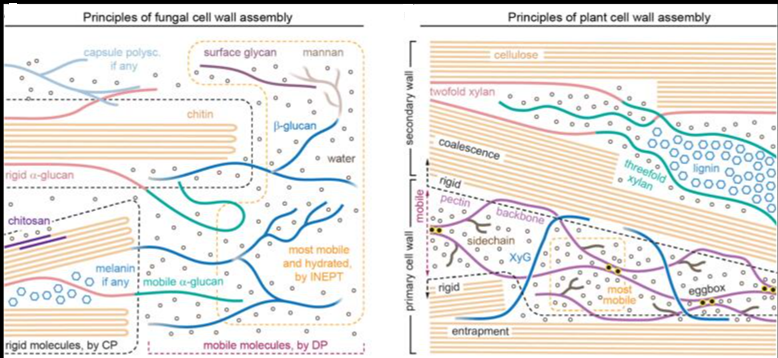
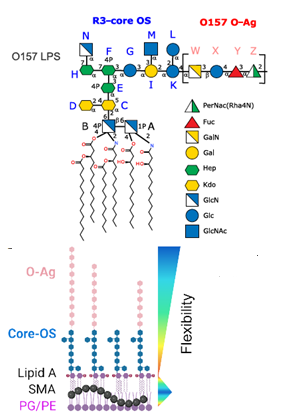
The primary sequence of a biopolymer encodes the essential folding information that allows it to perform sophisticated functions. Inspired by natural biopolymers, peptide and nucleic acid sequences have been designed to adopt specific three-dimensional (3D) shapes and programmed to perform specific functions. In contrast, synthetic glycans capable of autonomously folding into defined 3D conformations have not been explored due to their structural complexity and lack of design rules. Here, we generate a glycan that adopts a stable secondary structure not found in nature, a glycan hairpin, by combining natural glycan motifs stabilized by an unconventional hydrogen bond and hydrophobic interactions. Automated glycan assembly allowed rapid access to synthetic analogues, including site-specific 13C-labeled ones, for nuclear magnetic resonance conformational analysis.
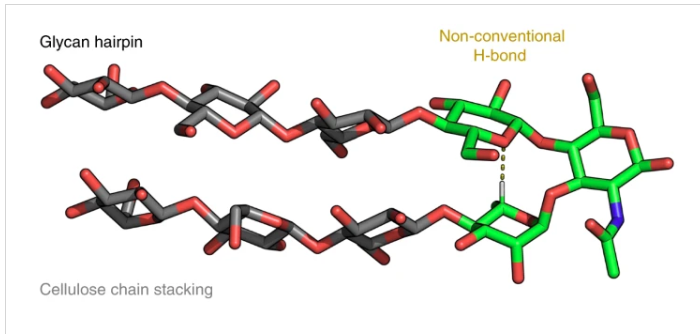
Long-range inter-residue nuclear Overhauser effects confirmed the folded conformation of the synthetic glycan hairpin. Controlling the 3D shape over the pool of available monosaccharides can provide more foldamer scaffolds with programmable properties and functions.