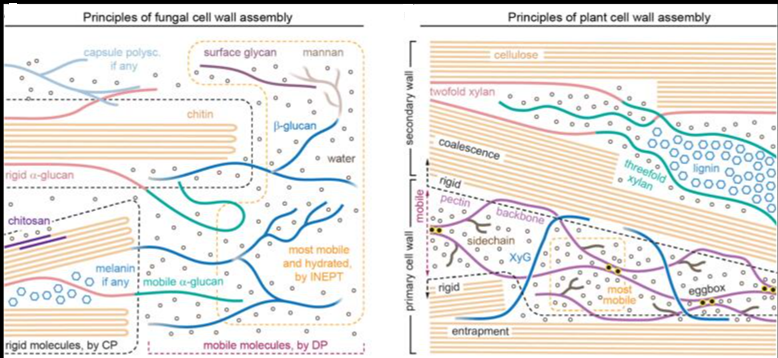
SARS-CoV-2 employs heparan sulfate (HS) as an initial cellular attachment factor, and therefore, there is interest in developing heparin as a therapeutic for SARS-CoV-2. The authors describe the preparation of well-defined heparin mimetics by a controlled head-to-tail assembly of Heparan Sulfate oligosaccharides having an alkyne or azide moiety by copper-catalyzed azide-alkyne cycloaddition (CuAAC). Alkyne- and azide-containing sulfated oligosaccharides were prepared from a common precursor by modifying an anomeric linker with 4-pentynoic acid and by enzymatic extension with an N-acetylglucosamine having an azide moiety at C-6 (GlcNAc6N3), respectively, followed by CuAAC. The process of enzymatic extension with GlcNAc6N3 followed by CuAAC with the desired alkyne-containing oligosaccharides could be repeated to give compounds composed of 20 and 27 monosaccharides, respectively.
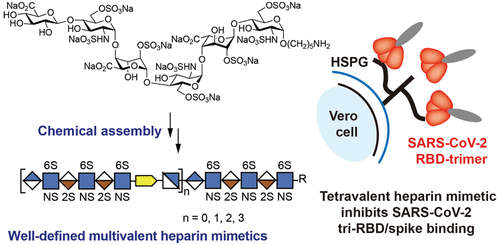
The heparin mimetics could inhibit the SARS-CoV-2 spike or RBD binding to immobilized heparin or to Vero E6 cells. The inhibitory potency increased with increasing chain length, and a compound composed of four sulfated hexasaccharides linked by triazoles had a similar potency as unfractionated heparin. Sequence analysis and HS microarray binding studies with a wide range of RBDs of variants of concern indicate that they have maintained HS-binding capabilities and selectivities. The heparin mimetics exhibit no or reduced binding to antithrombin-III and platelet factor 4, respectively, associated with side effects.