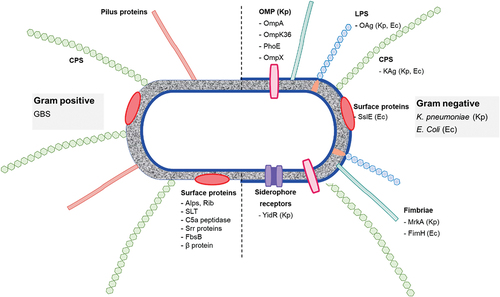
Infection of host cells by SARS-CoV-2 begins with recognition by the virus S (spike) protein of cell surface heparan sulfate, tethering the virus to the extracellular matrix environment and causing the subunit S1-RBD to undergo a conformational change into the ‘open’ conformation. These two events promote the binding of S1-RBD to the angiotensin-converting enzyme 2 (ACE2) receptor, a preliminary step toward viral-cell membrane fusion.
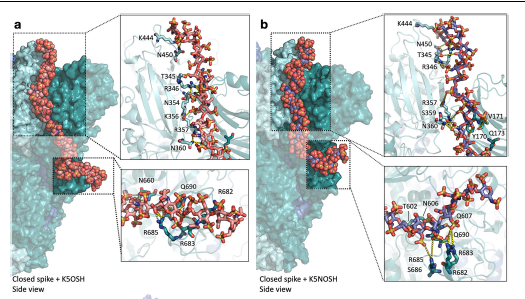
Combining ligand-based NMR spectroscopy with molecular dynamics, oligosaccharide analogues were used to explore the interactions between S1-RBD of SARS CoV-2 and HS, revealing several low specificity binding modes and previously unidentified potential sites for the binding of extended Heparan Sulfate polysaccharide chains.
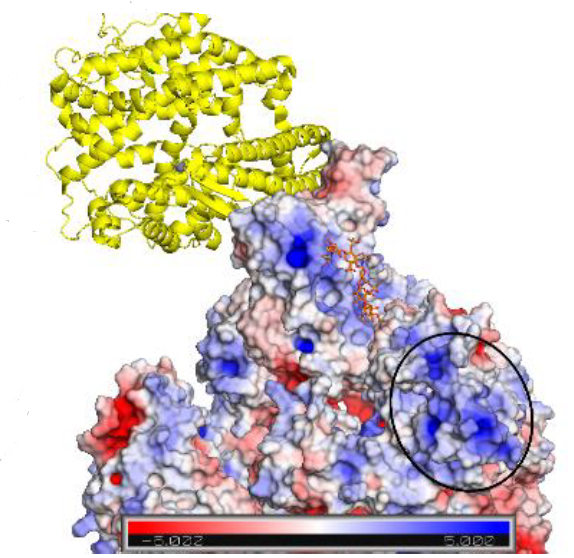
The evidence for multiple binding modes also suggests that particular inhibitors will not be optimal against protein S. Rather, diverse Heparan Sulfate -based structures, characterized by high affinity and multi-valent compounds, may be required.