Starch is the indispensable nutritive reserve of most green plants. Even though each plant species has a unique starch granular size, a conserved architecture of a constitutive series of organized levels is found. Starch is the most common carbohydrate in the human diet. As a prebiotic, resistant starch degradation, leading to glucose, requires the concomitant actions of specialized enzymes such as starch-binding proteins and hydrolases. The human gut symbiont Ruminococcus bromii expresses granular starch-binding protein: Starch Adherence System member 6 (Sas6) that consists of two starch-specific carbohydrate-binding modules from family 26 (RbCBM26) and family 74 (RbCBM74). The authors report the results of a crystallographic investigation of RbCBM74 in its interactions with a double helical dimer of maltododecaose bound along an extended surface groove. Binding data combined with native mass spectrometry suggest that RbCBM26 binds short maltooligosaccharides while RbCBM74 can bind single and double helical alpha 1-4 linked glucans.
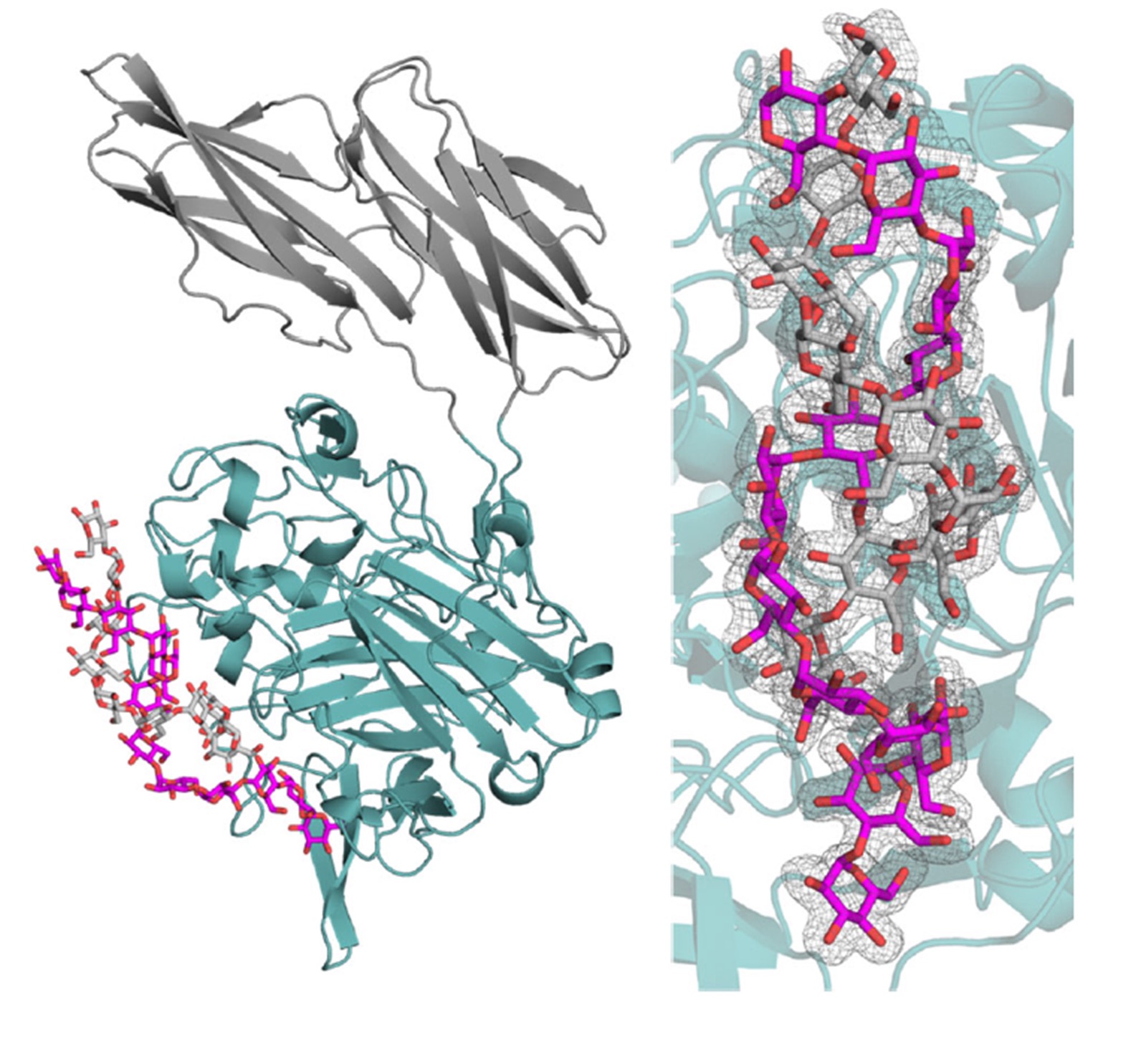
(PDB : 7UWU, 7UWV; 7UWW)




