Naturally occurring plant cellulose, the most abundant renewable resource consists of fibers of long polymer chains tightly packed in parallel arrays in either of two crystal phases collectively referred to as cellulose I. During mercerization, a process that involves treatment with sodium hydroxide, cellulose converts to another crystal form called cellulose II, within which every other chain has remarkably changed direction.
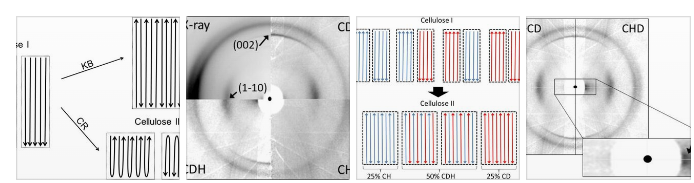
The authors designed a neutron diffraction experiment with deuterium labelling to understand how this change of cellulose chain direction is possible. They show that during the mercerization of bacterial cellulose, chains fold back on themselves in a zigzag pattern to form crystalline anti-parallel domains. This result provides a molecular-level understanding of one of the most widely used industrial processes for improving cellulosic materials.




