Many pathogens exploit host cell-surface glycans. However, precise analyses of glycan ligands binding with heavily-modified pathogen proteins can be confounded by overlapping sugar signals and/or compound with known experimental constraints. ‘Universal saturation transfer analysis’ (uSTA) builds on existing nuclear magnetic resonance spectroscopy to provide an automated workflow for quantitating protein-ligand interactions.
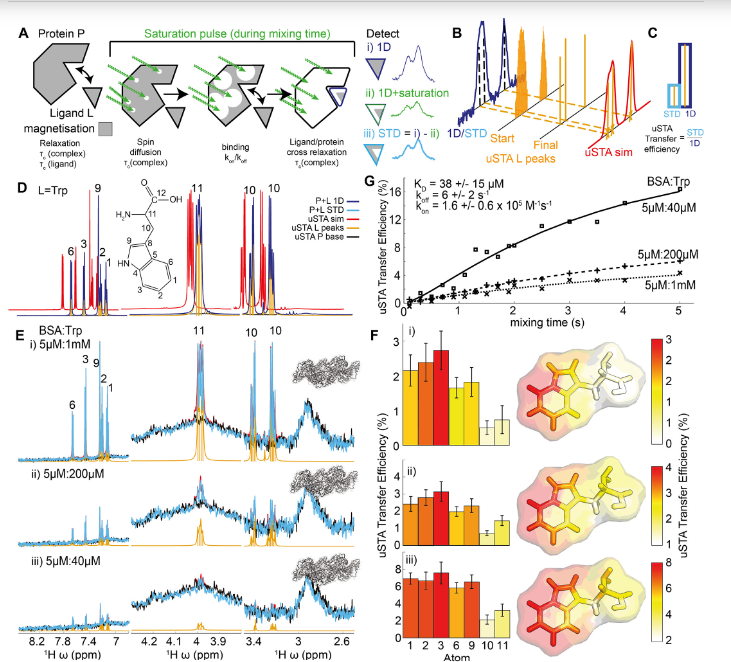
uSTA reveals that early-pandemic, B-origin lineage SARS-CoV-2 spike trimer binds sialoside sugars in an ‘end-on’ manner. uSTA-guided modelling and a high-resolution cryo-electron microscopy structure implicate the spike N-terminal domain (NTD) and confirm end-on binding. This finding rationalizes the effect of NTD mutations that abolish sugar-binding in SARS CoV 2 variants of concern. Together with genetic variance analyses in early pandemic patient cohorts, this binding implicates a sialylated polylactosamine motif found on tetraantennary N-linked glycoproteins in deeper human lung as potentially relevant to virulence and/or zoonosis.