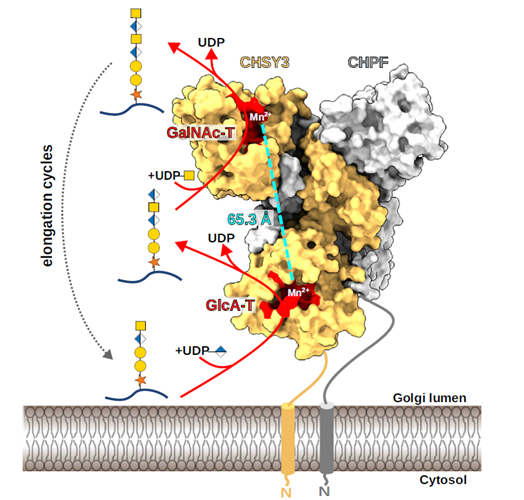
Glycosaminoglycans (GAGs) are complex linear polysaccharides covalently attached to core proteins (except for hyaluronan), where they form proteoglycans. They play vital roles in the organization of the extracellular matrix and at the cell surface, where they contribute to cell signalling and adhesion regulation. To explore the mechanisms and pathways underlying their functions, the authors generated an expanded dataset of 4,290 interactions corresponding to 3,464 unique GAG-binding proteins, four times more than the first version of the GAG interactome (Vallet, Clerc, and Ricard-Blum. J Histochem Cytochem 69: 93–104, 2021).
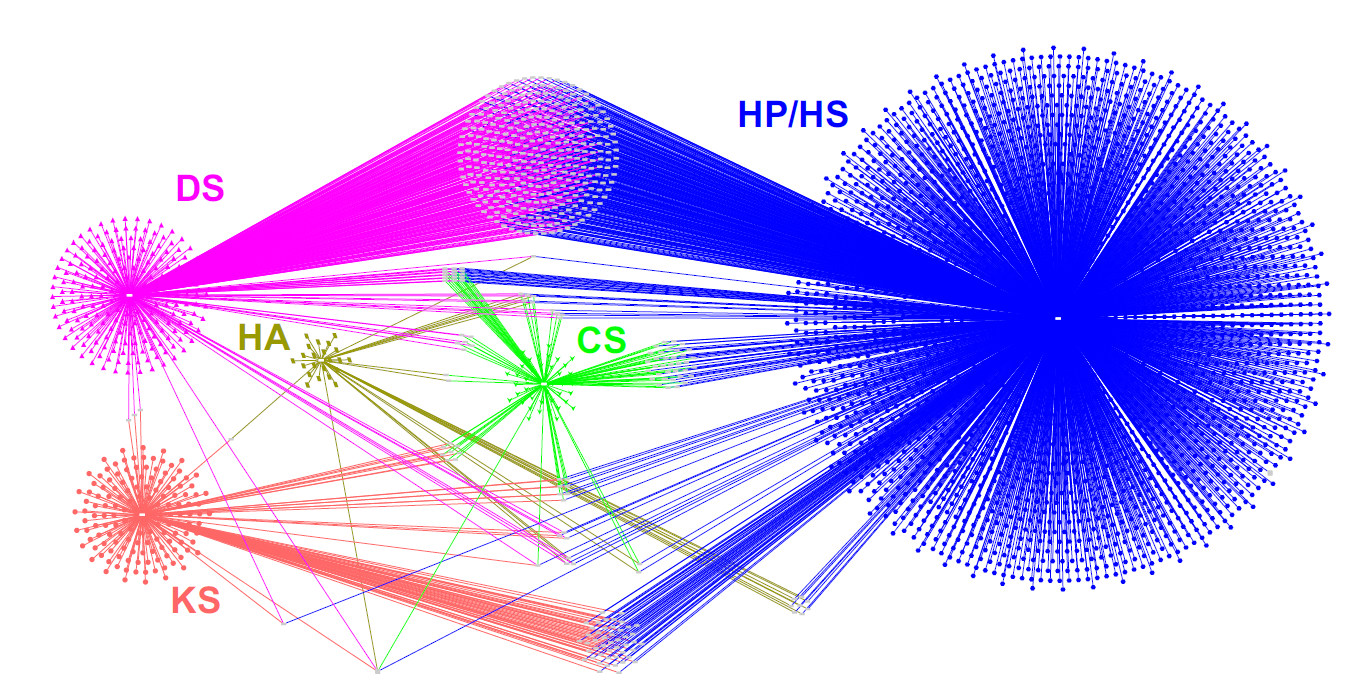
The increased size of the GAG network is primarily due to the addition of GAG-binding proteins captured from cell lysates and biological fluids by affinity chromatography and identified by mass spectrometry. The authors review the interaction repertoire of natural GAGs and synthetic sulfated hyaluronan, the specificity and molecular functions of GAG-binding proteins, and the biological processes and pathways they are involved in. This dataset also investigates the differences between proteins binding to iduronic acid-containing GAGs (dermatan sulfate and heparin/heparan sulfate) and those interacting with GAGs lacking iduronic acid (chondroitin sulfate, hyaluronan, and keratan sulfate).