Carbohydrate-binding proteins from pathogenic bacteria and fungi participate in various pathological processes, where they interact with glycan present on the surface of the host cells. These interactions are part of the host’s initial infection processes; their investigations are fundamental to be performed at the atomic level. The article reports a carbohydrate-binding protein’s first neutron structure in complex with a fully deuterated carbohydrate ligand. The PLL lectin’s structural elucidation from Photorhabdus laumondii has been conducted under Neutron diffraction at room temperature at the Institut Laue Langevin in Grenoble.
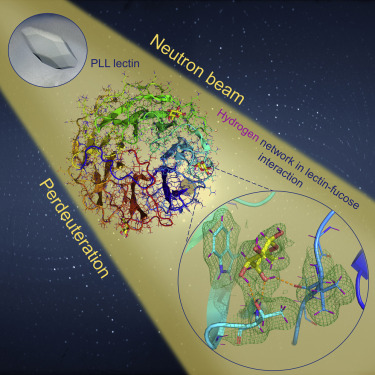
Both the apo form and the complex with deuterated L-fucose are reported. A detailed structural analysis of the lectin-carbohydrate interactions provides unique information on the hydrogen bond network, the role of water molecules, and the extent of the CH-Ï€ stacking interactions between fucose and the aromatic amino acids in the binding site.




