Glycans are involved in various life processes and represent critical targets of biomedical developments. Nevertheless, the accessibility to long glycans with precise structures remains challenging. The article reports on the synthesis of glycans consisting of [→4)-α-L-Rhap-(1→3)-β-D-Manp-(1→] repeating unit. The glycan is relevant to the O-antigen of Bacteroides vulgatus, a common component of gut microbiota. The optimal combination of assembly strategy, protecting group arrangement, and glycosylation reaction have enabled the synthesis up to a 128-mer glycan.
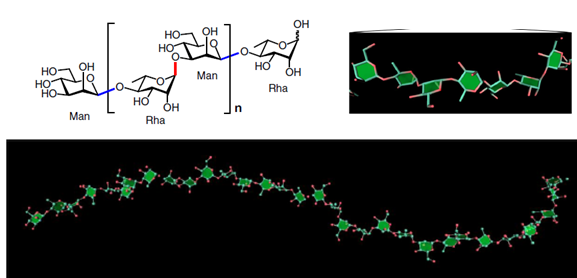
The synthetic glycans are accurately characterized by advanced NMR and MS approaches, the 3D structures are defined, and their potent binding activity with human DC-SIGN, a receptor associated with the gut lymphoid tissue, is disclosed.




