Both DC-SIGN and langerin are lectins of type II with C-type CRDs. The sequences and the overall structures of their CRDs are close (fig. 22) and correspond to the classical CRD fold However, several important differences can be tracked in these two CRDs.
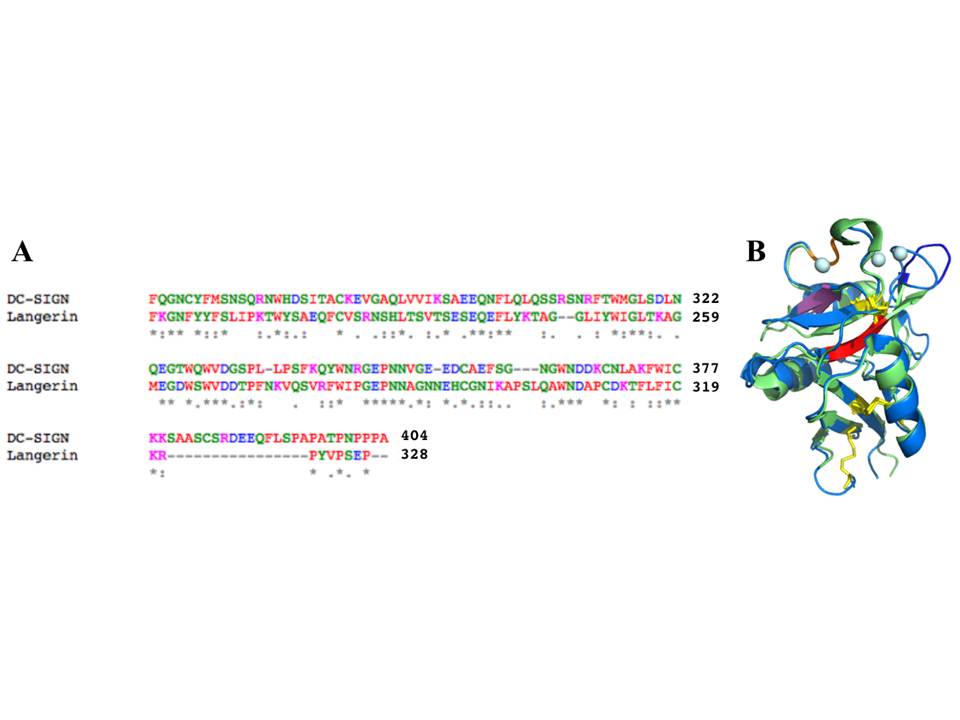
A, An alignment of the sequences of DC-SIGN and langerin CRDs by ClustalW (amino acid color codes: red – hydrophobic, green – polar, pink – positively charged, blue – negatively charged). B, structural alignment of DC-SIGN and langerin CRDs (the color coding is the same as in fig. 2.9 and 2.18)
Langerin has several structural elements that are not present in DC-SIGN CRD, including a 310 helix close to sugar-binding site, and an additional b2´ strand (fig. 18). The whole CRD structure of langerin is supported by two conserved disulphide bridges, in contrast to four S-S bonds in DC-SIGN. Unlike DC-SIGN, langerin CRD has only one Ca2+ ion at Ca site 2 (conventional sugar-binding site), and the lack of other Ca2+ ions in sites 1 and 3 might be the reason for the high flexibility of the b2-b2´ loop comprising residues 258-262, which also leads to the formation of a large groove specific to the langerin structure.
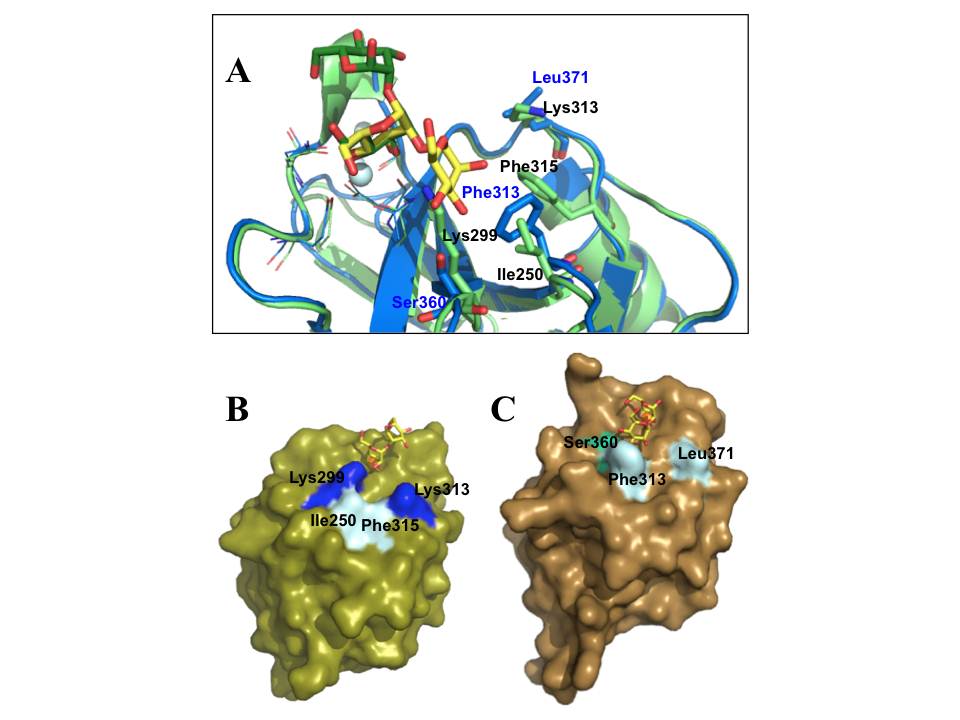
A, The alignment of DC-SIGN (blue) (pdb:2IT5) and langerin (light green) (pdb:3P5F) CRDs bound to Mana1- 2Man, shown in yellow (for DC-SIGN) and green (for langerin) sticks; the side chains of EPN and WND motifs are shown in lines, and the most important different amino acid residues required for sugar binding are highlighted by stick representation; the blue and black labels correspond to DC-SIGN and langerin side chains, respectively; light cyan spheres are Ca2+ ions. B and C are surface representations of langerin and DC-SIGN CRDs, respectively, complexed with Mana1-2Man; the important side chains are highlighted; green sphere is Ca2+.
However, the most relevant difference of these two lectins is in their sugar-binding site topologies. A unique feature of langerin CRD is the presence of two lysine residues (Lys299 and Lys313) in sugar binding-site, which provide the binding site the basic character that allows langerin to accommodate sulfated sugars in its binding site. On the other hand, while Phe313 is an important side chain in DC-SIGN sugar-binding site as it forms stacking interactions with the sugar ring (fig. 23A) (Drickamer, K. 1999, Feinberg, H, Mitchell, D. A, Drickamer, K, & Weis, W. I. 2001) in langerin Phe315 positioning is not suitable to participate in such sugar binding (fig. 23A). Overall topology of langerin CRD composes only a small binding site strongly constrained by Lys299 (fig. 23B), while DC-SIGN has a potential to adapt more extended oligosaccharides with a widely open binding site (fig. 23C and 9C).
The CRD-neck junctions of DC-SIGN and langerin are also different : while the flexibility in DC-SIGN is retained, langerin trimers present CRDs in a rather rigid manner (Feinberg, H, Powlesland, A. S, Taylor, M. E, & Weis, W. I. 2010). This likely leads to different way of ligand recognition of the two lectins.
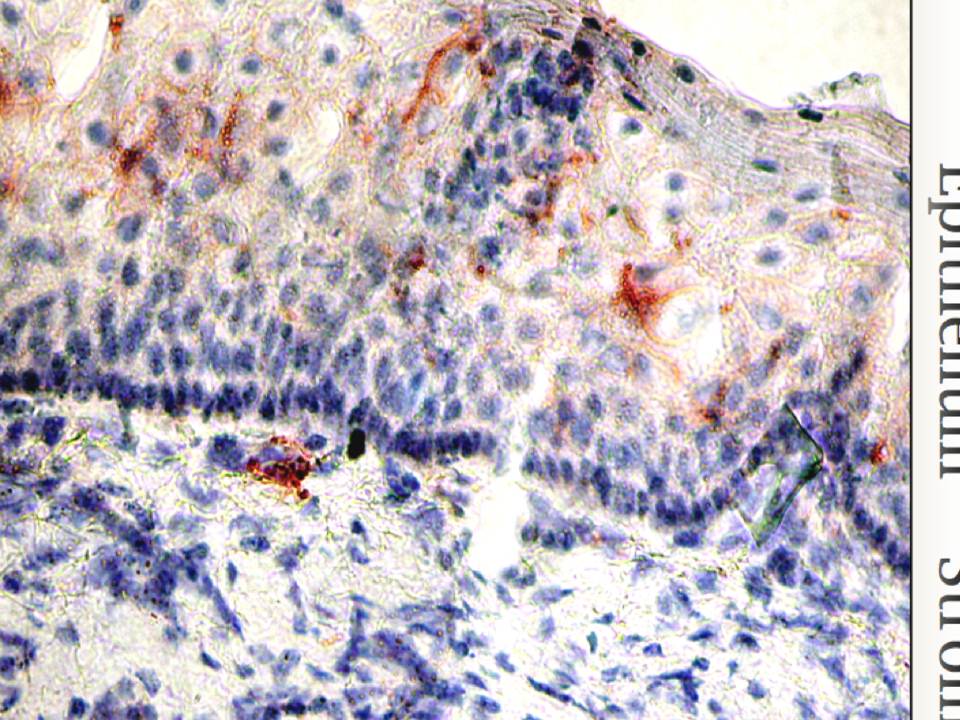
Figure 2.24 : The micrograph of mucosal tissue
Langerin is visualized in red, cell nuclei are in blue.
Finally, the localization of these two lectins in mucosa is different. Langerin is expressed on LCs that reside in epithelia (fig. 24), while DC-SIGN expressing stromal DCs survey the underlying stroma. Thus LCs with langerin are the very first barrier that pathogens encounter invading the mucosal tissues.
